Disposable sterile syringe with sheath included
A sheath tube and syringe technology, applied in the field of medical equipment, can solve the problems of needle pollution, accidental needle stick injury, contaminated needles, etc., achieve good biodegradability, avoid cross-infection, and good tensile properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
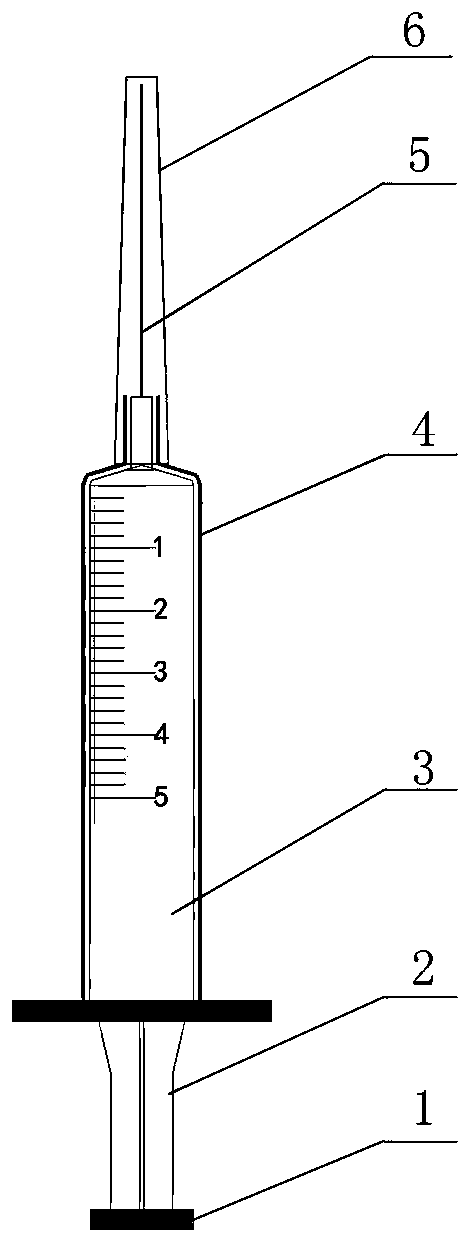
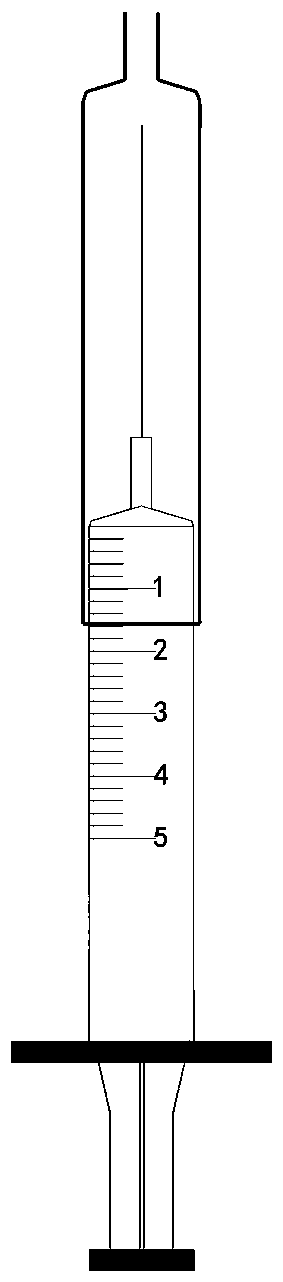
[0023] A disposable sterile syringe comprising a sheath tube, which includes a handle 1, a push rod 2, a syringe 3, a protective sheath 4, a needle 5 and a trocar cap 6; the needle 5 is integrated with the syringe 3 and cannot be separated, and the This can avoid the needle stick injury caused in the process of separating the needle; the outer sleeve 4 is located outside the syringe 3; when the liquid medicine is sucked, there is no need to return the needle cap to protect the needle, but push the outer sleeve toward the needle , push it until it can cover the entire needle, and then inject after the nurse prepares the patient's position and skin disinfection. At this time, you only need to push the outer cannula in the opposite direction until the needle is completely exposed, which is conducive to protection. The needle is in a sterile state before injection, which is also beneficial to avoid needle stick injury caused by the nurse's direct contact with the needle during the ...
Embodiment 2
[0025] An outer sleeve, which is prepared from the following raw materials in parts by weight: 20 parts of polybutylene succinate, 15 parts of polyvinyl alcohol, 8 parts of hydroxymethyl cellulose, 7 parts of polyglycolic acid, chitosan 6 parts, 4 parts of polylactic acid, 3 parts of barium stearate, 2 parts of mica powder, 2 parts of corn starch, 1 part of sodium succinate and 1 part of silica gel, the pore volume of the silica gel is 0.60-0.85ml / g, the average The pore size is 4.5-7.0nm, and the specific surface area is 450-650m 2 / g; the particle diameters of the hydroxymethylcellulose, chitosan, barium stearate, mica powder, cornstarch and sodium succinate are all controlled at 100 mesh.
[0026] The preparation method of the above-mentioned outer casing comprises the following steps: taking each raw material according to the weight part for later use; first adding each raw material into the mixer in turn, stirring centrifugally at 1000 rpm for 3 minutes, and then standing...
Embodiment 3
[0028] An outer sleeve, which is prepared from the following raw materials in parts by weight: 22 parts of polybutylene succinate, 16 parts of polyvinyl alcohol, 10 parts of hydroxymethyl cellulose, 8 parts of polyglycolic acid, chitosan 7 parts, 5 parts of polylactic acid, 4 parts of barium stearate, 3 parts of mica powder, 3 parts of corn starch, 2 parts of sodium succinate and 2 parts of silica gel, the pore volume of the silica gel is 0.60-0.85ml / g, the average The pore size is 4.5-7.0nm, and the specific surface area is 450-650m 2 / g; the particle diameters of the hydroxymethylcellulose, chitosan, barium stearate, mica powder, cornstarch and sodium succinate are all controlled at 150 mesh.
[0029] The preparation method of the above-mentioned outer casing comprises the following steps: taking each raw material according to the weight part for later use; first adding each raw material into the mixer in turn, stirring centrifugally at 1000 rpm for 3 minutes, and then stand...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com