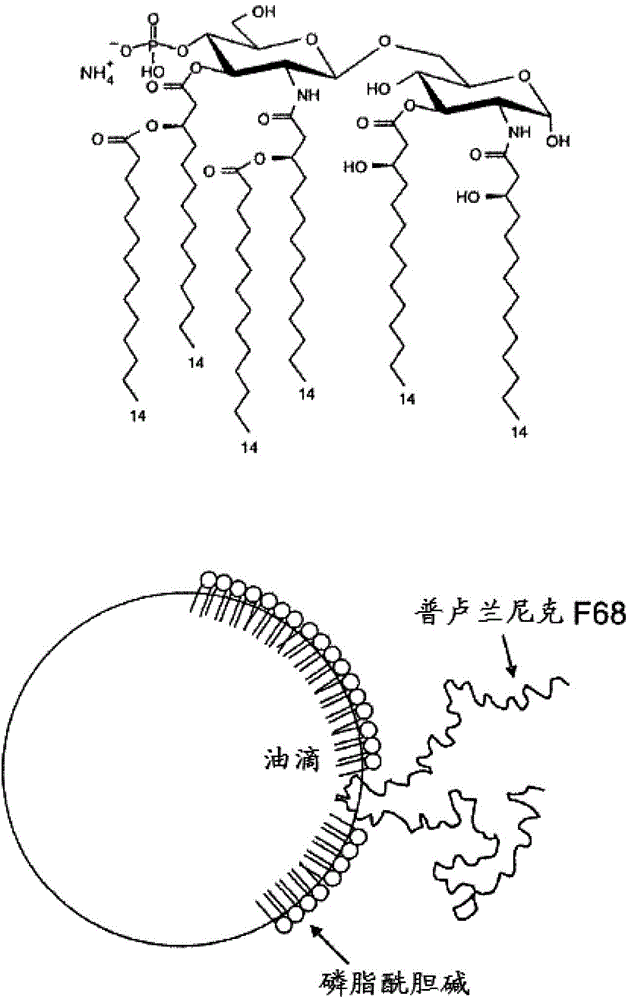
Vaccines for HSV-2
A HSV-2, immunogenic technology, applied in the direction of medical preparations containing active ingredients, allergic diseases, peptide sources, etc., can solve problems such as unsuccessful efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
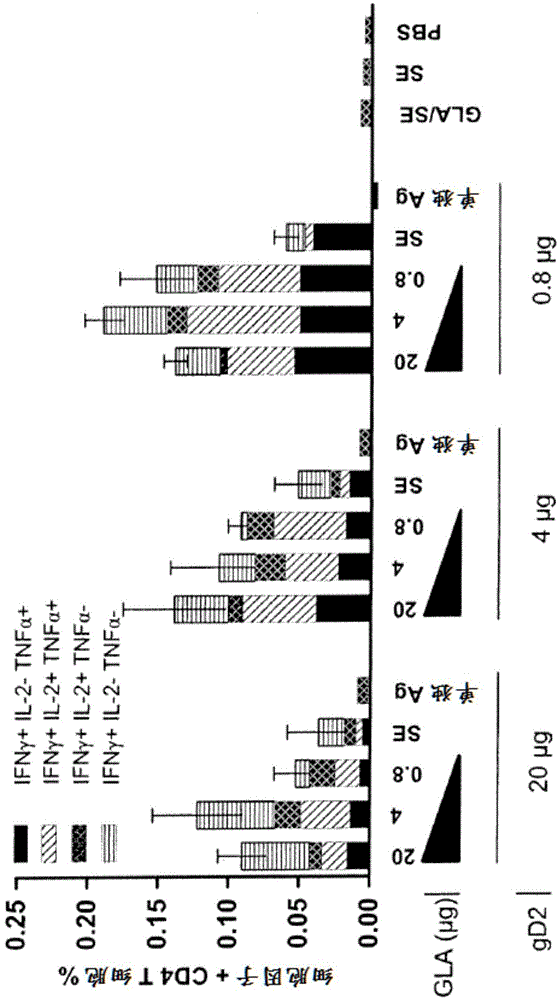
[0393] Increased CD4 T cell-based immunity against HSV-2 GD2 protein occurs when formulated with the adjuvant GLA-SE after multiple vaccinations in mice
[0394] In this example, the ability of GLA-SE to increase CD4 T cell responses following immunization of mice with a recombinant protein vaccine was assessed.
[0395] 0.8, 4 or 20 μg in combination with 0.8, 4 or 20 μg GLA-SE (SE percentage was 2% in this study and all subsequent studies), SE alone or PBS delivered intramuscularly in 100 μl (50 μl per leg) Five Balb / c mice in each group were immunized with recombinant gD protein via a prime / boost immunization protocol (d0 prime / d21 boost). Mice immunized with GLA-SE, SE alone or PBS in the absence of recombinant protein served as negative controls. On day 4 post-boost, by using gD 272-285 Antigen-specific splenic CD4 T cell responses were measured by intracellular cytokine staining (ICS) for IFN-γ, TNF-α, and IL-2 following ex vivo restimulation of splenocyte cultures wit...
Embodiment 2
[0397] GLA increases CD8 T cell responses in mice
[0398] In this example, the ability of GLA-SE to increase CD8 T cell responses following immunization of mice with a recombinant protein vaccine was assessed.
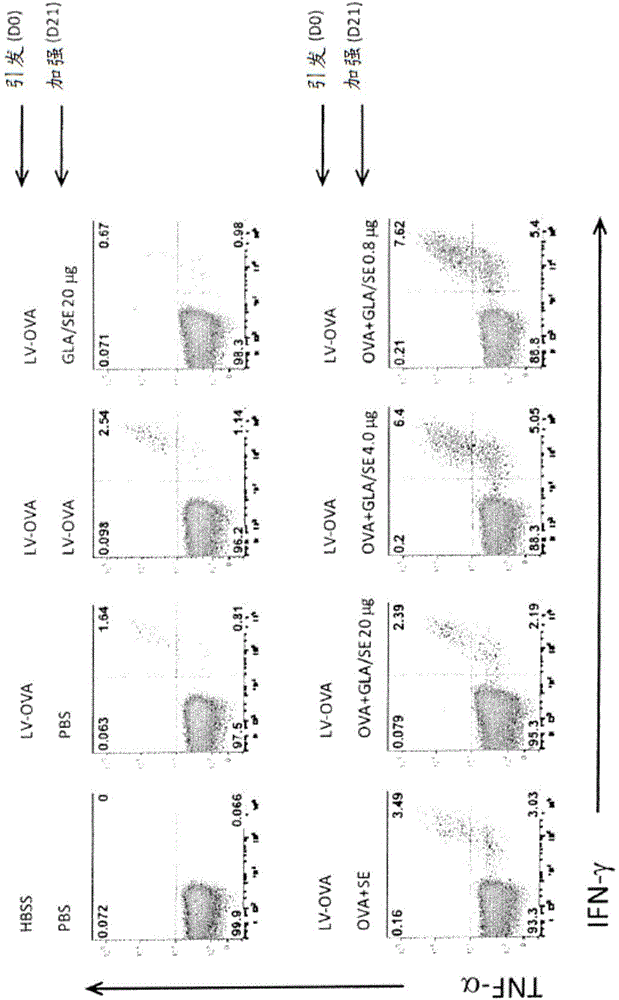
[0399] Ovalbumin was used as a model protein. Female C57Bl / 6 mice were injected s.c. with ovalbumin encoding lentivirus ( image 3 and 4 "LV-OVA" in ) and on day 21 by i.m. injection of recombinant ovalbumin adjuvant with various doses of GLA-SE ( image 3 and 4"OVA+GLA / SE") in the Four days later, splenic T-cell responses to the following in vitro stimulators were measured by intracellular cytokine staining (ICS): antibodies to OVA MHC class I peptides 55-62 and 257-264 and MHC class II peptides 323-339 or CD3 and CD28 . CD8 T cells are defined as cells that secrete any of the cytokines IFN-γ, IL-2, and TNF-α
[0400] Such as image 3 As shown in , there was a higher percentage of CD8 T cells in mice that received an antigen boost, with the highest percentage...
Embodiment 3
[0402] CD4 T cell-based immunogenicity against individual HSV-2 GD2, UL19 and UL25 proteins following multiple vaccinations in mice
[0403] The goal of this set of studies was to identify a single mouse strain in which CD4 T cell-based immunogenicity against each protein subunit in the vaccine could be assessed. To this end, a series of experiments were performed in mice to identify d ), C57BL / 6(H-2 b ) and CB6F1 (H-2 d +2 b ))) individual CD4 T cell epitopes within each HSV-2 antigen (ie gD2, UL19 and UL25). The experimental strategy consisted of intramuscular immunization of uninfected mice in 100 μl (50 μl per leg) with 5 μg of each recombinant protein formulated together with 5 μg GLA-SE as monovalent immunogen within the context of a prime / boost immunization protocol (d0 prime / d21 boost). rat composition. Antigen-specific CD4 T cell responses were analyzed on day 4 post-boost using 15-mer peptide libraries whose sequences were derived from the corresponding amino ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com