Cervical vertebra interbody fusion cage
A technology of intervertebral fusion device and fusion device, which is applied in the field of cervical intervertebral fusion device, to achieve the effect of reducing discomfort and avoiding the removal of secondary surgery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
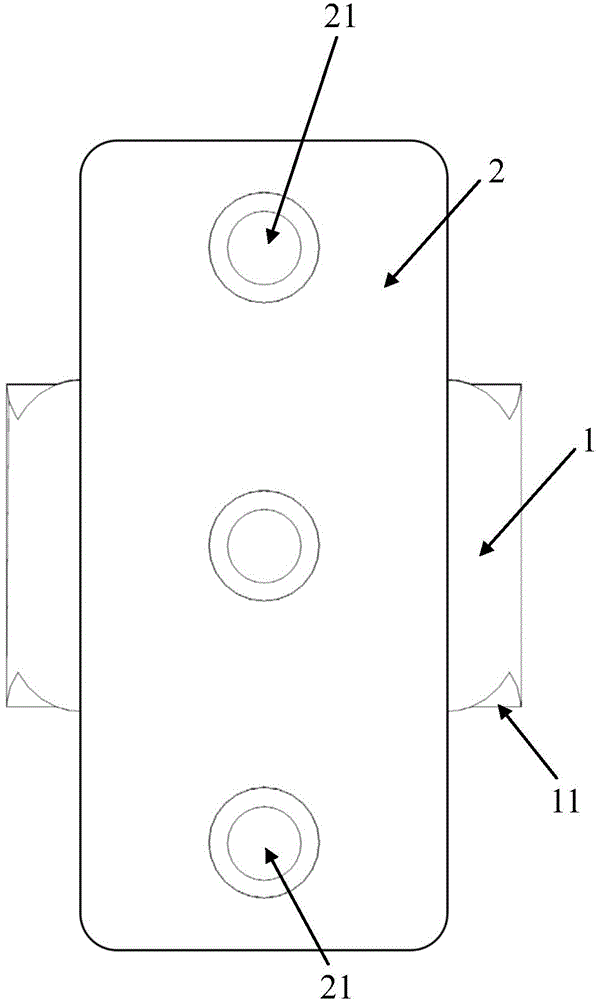
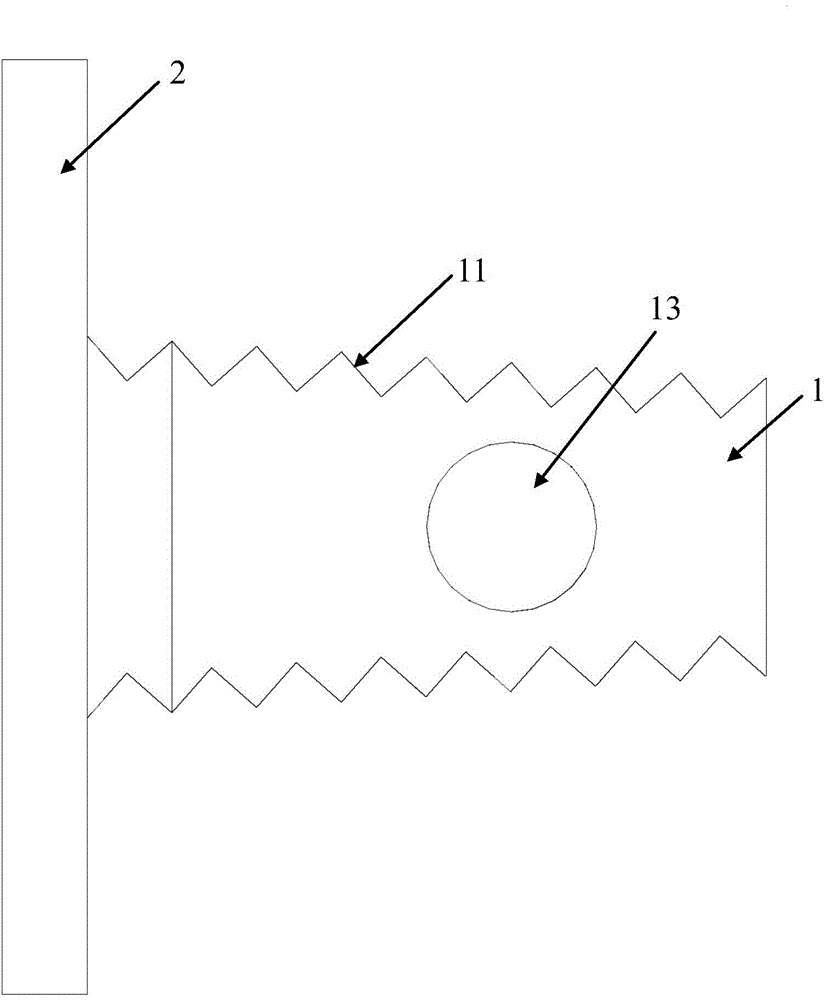
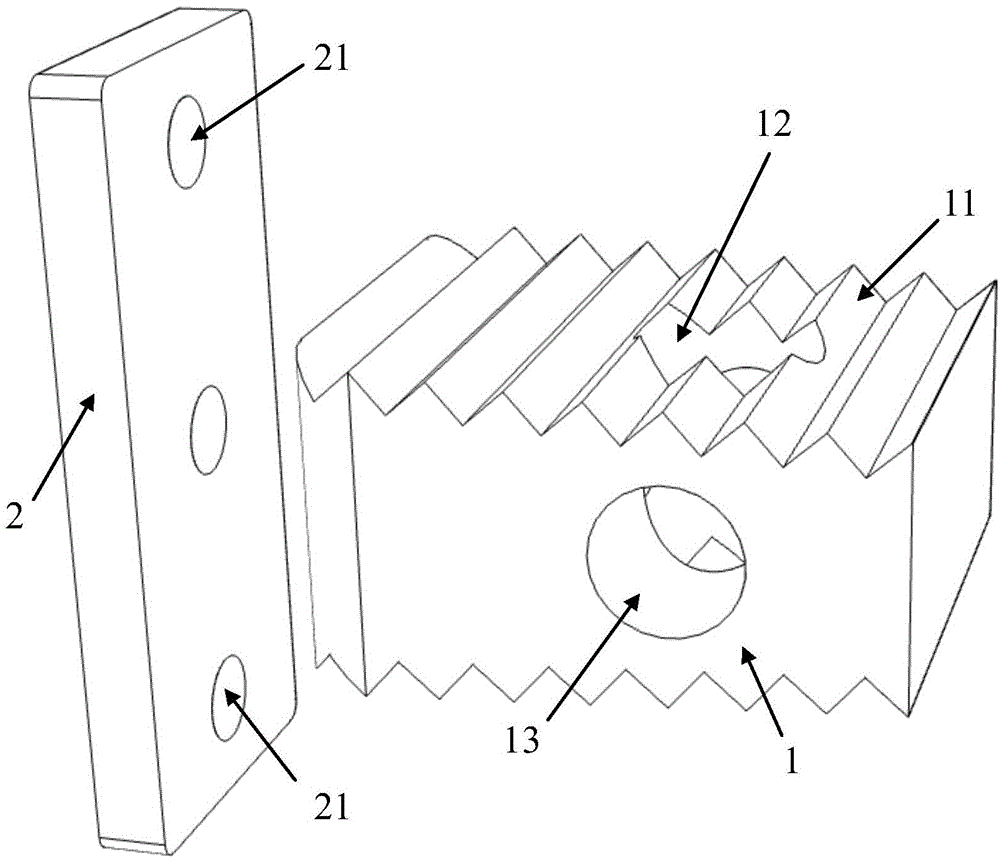
[0047] This embodiment is used to prepare a cervical intervertebral fusion device made of PLA material. Among them, the fuser body 1 is made of pure L-lactic acid (PLLA, with a molecular weight of 800,000) material; the size of the fuser body 1 is 14.0 mm in length, 12.5 mm in width, 7.0 mm in height at the front end, and 6.0 mm in height at the rear end; The upper and lower end surfaces of the fusion device main body 1 have a tooth-like structure with a depth of 0.35 mm and a spacing of 2.0 mm; the left and right sides of the fusion device main body 1 are centered near the rear and have a cylindrical vertical through hole 12 with a diameter of 4.6 mm. The center of the vertical through hole 12 reaches The distance between the rear end surface of the fuser main body 1 is 6.3mm. After testing, the axial compressive strength of the cage body 1 of this embodiment is 110.6 MPa.
[0048] The anterior cervical fixation plate 2 is made of pure racemic polylactic acid (PDLLA, with a ...
Embodiment 2
[0051] This embodiment is used to prepare the cervical intervertebral fusion device of PLA / PCL composite material. Among them, fusion device main body 1 is made of pure PCL (molecular weight: 150,000) material; fusion device main body 1 is 15.0 mm long, 12.0 mm wide, front end height is 8.0 mm, rear end height is 7.0 mm; fusion device main body 1 The upper and lower end surfaces of the fusion device have a tooth-like structure with a depth of 0.5 mm and a spacing of 2.0 mm; the left and right sides of the fusion device body 1 are centered near the back and have a square vertical through hole 12 with a side length of 5.5 mm, and the center of the vertical through hole 12 reaches the fusion device body 1 The distance of the rear end face is 7.0 mm. After testing, the axial compressive strength of the cage body 1 of this embodiment is 121.5 MPa.
[0052] The anterior cervical fixation plate 2 is made of pure left-handed polylactic acid (PLLA, with a molecular weight of 200,000);...
Embodiment 3
[0055] This embodiment is used to prepare the cervical intervertebral fusion device common to the PLGA / β-TCP composite material and the PCL / β-TCP composite material. Among them, the fuser main body 1 is made of PCL / β-TCP composite material (PCL molecular weight is 176,000, β-TCP particle size distribution is 12.0±2.0 μm), PCL / β-TCP=6 / 4(w / w) ; The size of fusion device main body 1 is 16.0 mm in length, 14.0 mm in width, 9.0 mm in height at the front end, and 7.5 mm in height at the rear end; the upper and lower end surfaces of the fusion device body 1 have tooth-like structures with a depth of 0.6 mm and a spacing of 2.2 mm ; There is a cylindrical vertical through hole 12 with a diameter of 6.0 mm in the left and right of the fusion device body 1 near the back, and the distance from the center of the vertical through hole 12 to the rear end surface of the fusion device body 1 is 8.0 mm. After testing, the axial compressive strength of the cage body 1 of this embodiment is 137....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Width | aaaaa | aaaaa |
| Height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com