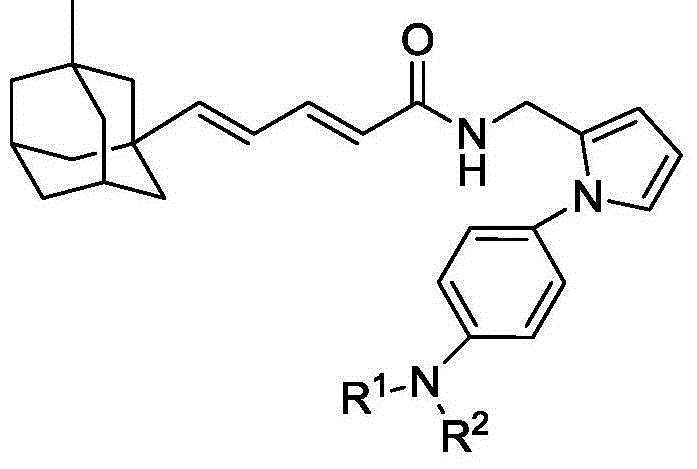
Compound containing aniline and diene adamantane structure and preparation method and application thereof
A compound and diene technology, applied in the field of drugs related to thrombotic diseases, can solve problems such as high bleeding risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025]
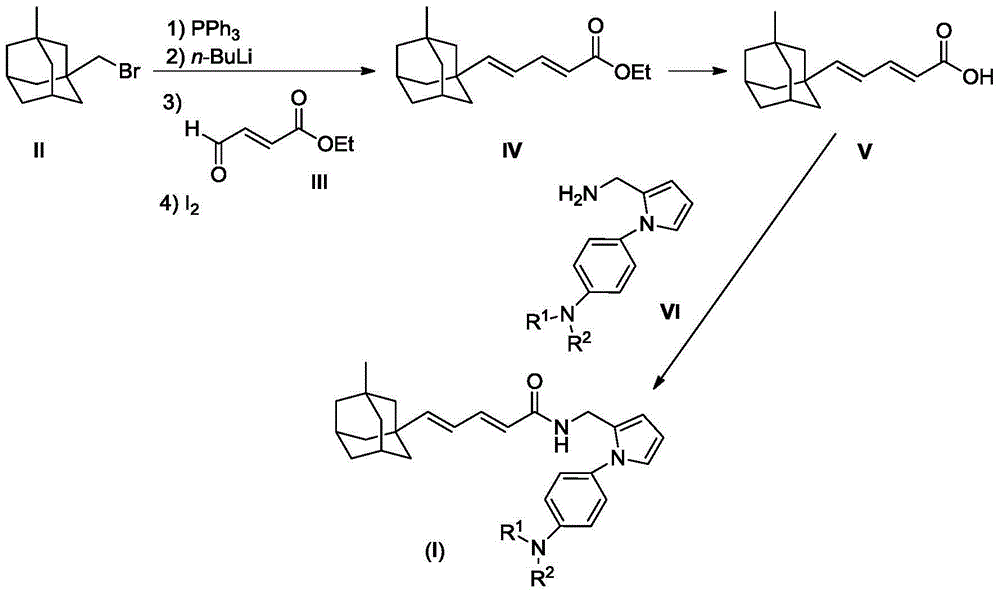
[0026] 2.43g (10mmol) compound II and 2.62g (10mmol) PPh 3 Dissolve in 20mL dry THF, reflux overnight under nitrogen protection. After the reaction mixture was cooled to room temperature, a white turbid solution was obtained. Under the protection of nitrogen, it was cooled to -78°C, and 6.25 mL (10 mmol, 1.6M) n-BuLi in n-hexane was slowly added dropwise. After the addition is complete, continue to stir for one hour, and then add dropwise a solution of compound 1.28g (10mmol) III dissolved in 2mL THF. After the addition was completed, the reaction mixture was slowly warmed to room temperature, and then refluxed for 1 hour. The reaction mixture was poured into ice water, stirred, and extracted with 50 mL×3 dichloromethane. The combined extracts were washed with brine, and dried over anhydrous sodium sulfate. The desiccant was removed by suction filtration, 0.50 g of iodine was added to the filtrate, and the mixture was stirred overnight at room temperature. The react...
Embodiment 2-4
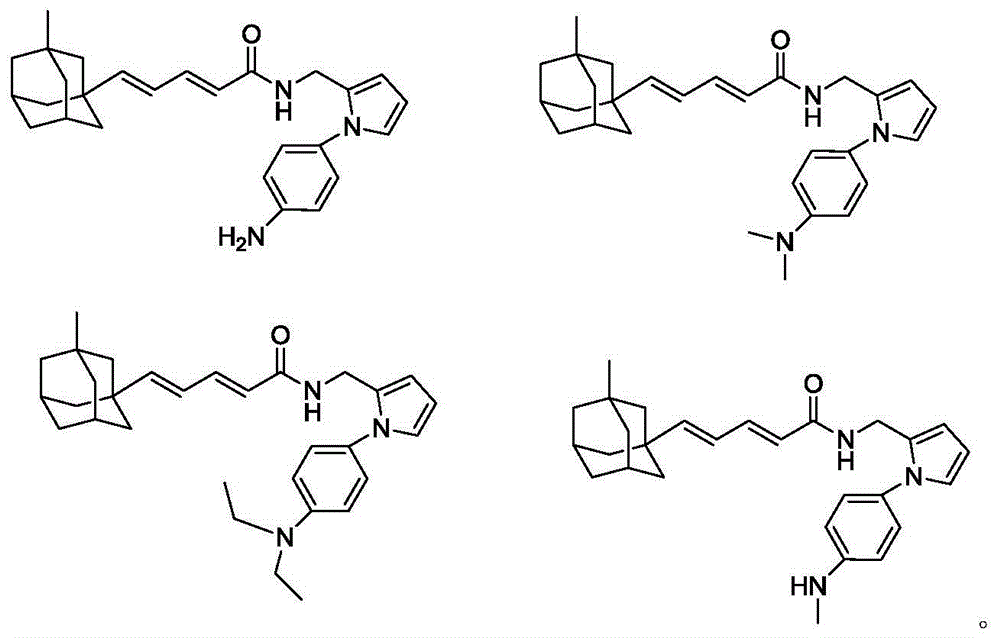
[0030] With reference to the method of Example 1, the following compounds with general formula I were synthesized.
[0031]
[0032]
Embodiment 5
[0033] Example 5 Preparation of reference compound D1
[0034] In order to fully illustrate the beneficial effects of the compounds of the present invention, the applicant recorded the compound D1 (unpublished) found during the experiment as a reference compound for pharmacodynamics.
[0035]
[0036] The synthesis method is as follows:
[0037]
[0038] 2.43g (10mmol) compound II and 2.62g (10mmol) PPh 3 Dissolve in 20mL dry THF, reflux overnight under nitrogen protection. After the reaction mixture was cooled to room temperature, a white turbid solution was obtained. Under the protection of nitrogen, it was cooled to -78°C, and 6.25 mL (10 mmol, 1.6M) n-BuLi in n-hexane was slowly added dropwise. After the addition is complete, continue to stir for one hour, and then add dropwise a solution of compound 1.28g (10mmol) III dissolved in 2mL THF. After the addition was completed, the reaction mixture was slowly warmed to room temperature, and then refluxed for 1 hour. The reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com