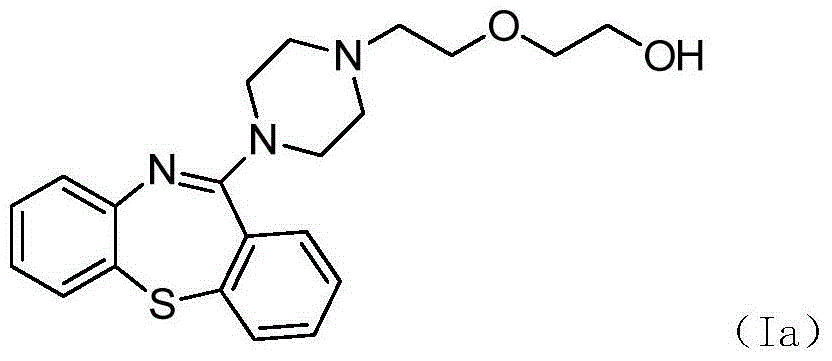
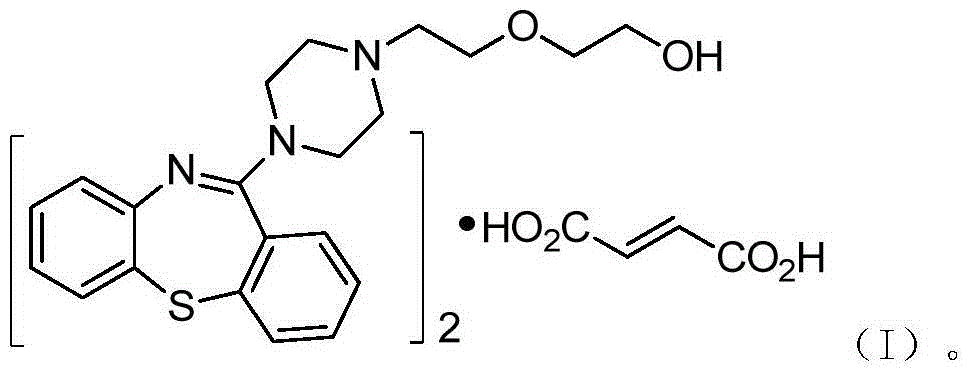
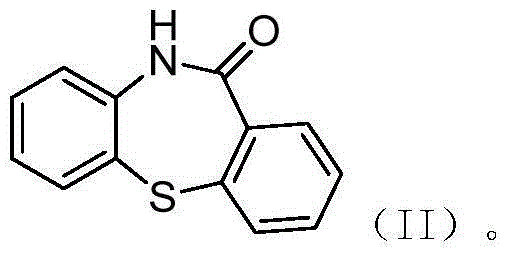
Preparation method of quetiapine intermediate
A technology of intermediates and thiazepines, applied in the field of medicinal chemistry, can solve problems such as loss of removal and transfer, difficulty in taking out phenyl groups, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0030] In order to enable those skilled in the art to better understand the technical solutions of the present invention, some non-limiting examples are further disclosed below to further describe the present invention in detail.
[0031] The reagents used in the present invention can be purchased from the market or can be prepared by the methods described in the present invention.
[0032] In the present invention, min means minute, h means hour, g means gram, ml means milliliter.
Embodiment 1
[0033] Example 1 10H-dibenzo[b,f][1,4]thiazepine Preparation of -11 Ketone
[0034] Add 3.00kg of water and 0.26kg of sodium hydroxide to the round bottom flask to control the temperature at 20°C, add 5.25kg of toluene and 1.22kg of 2-aminodiphenyl sulfide, and after the reaction solution is clarified, dissolve 1.04kg of phenyl chloroformate Add 1.00kg of toluene solution dropwise to it for 1.5h, keep warm for 3h after the dropwise addition, remove toluene by distillation under reduced pressure, add 10.06kg of polyphosphoric acid and 2.51kg of phosphorus pentoxide, raise the temperature to 90°C, react for 9h, then add 18.0kg of ice water and continue to cool down to 20°C and stir for 8h, filter to obtain a filter cake, beat with methanol and stir for 2h at room temperature, heat and crystallize at 0°C for 2h, filter, and vacuum dry at 60°C to obtain 1.21kg10H-dibenzo[b,f][ 1,4] Thiazepine -11 ketone, yield 95%, purity 99.391%.
Embodiment 2
[0035] Example 2 10H-dibenzo[b,f][1,4]thiazepine Preparation of -11 Ketone
[0036] Add 3.00kg of water and 0.26kg of sodium hydroxide to the round bottom flask to control the temperature at 30°C, add 5.25kg of toluene and 1.22kg of 2-aminodiphenyl sulfide, and after the reaction solution is clarified, dissolve 1.04kg of phenyl chloroformate Add 1.00kg of toluene solution dropwise to it for 1.5h, keep warm for 3h after the dropwise addition, add 8kg of water to wash once, remove toluene by distillation under reduced pressure, add 10.06kg of polyphosphoric acid and 2.51kg of phosphorus pentoxide, and heat up to 105 ℃, after reacting for 9 hours, add 18.0kg of ice water and continue to cool down to 25℃ and stir for 8h, filter to obtain a filter cake, beat with methanol and stir at room temperature for 2h, keep warm and crystallize at 0℃ for 2h, filter, and vacuum dry the filter cake at 60℃ to obtain 1.21kg 10H-dibenzo[b,f][1,4]thiazepine -11 ketone, yield 90.8%, purity 99.56...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com