Construction method of recombinant streptomyces lydicus for producing cellulase and natamycin
A technology of Streptomyces lydidae and cellulase, which is applied in the field of constructing recombinant Streptomyces lydidae producing cellulase and natamycin, which can solve the problems of narrow antibacterial spectrum, poor antibacterial ability and single antibacterial mechanism, etc. problem, to achieve the effect of improving the ability to degrade cellulose and improving the ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Embodiment 1, construct recombinant Streptomyces lydidii
[0089] 1. Preparation of DNA fragments
[0090] The synthetic nucleotide sequence is the DNA fragment of SEQ ID No.1 in the sequence listing. Among them, the DNA fragment shown in SEQ ID No.1, its name is P-vhb-glu, the DNA fragment from the 5' end to the 3' end is: the recognition site of Nde I, the coding sequence of Vitiligo hyaline hemoglobin , the recognition site of Xba I, the promoter of erythromycin resistance gene, the coding sequence of cellulase and the recognition site of EcoR I. SEQ ID No.1 is made up of 2140 nucleotides, the 1st-6th nucleotide of SEQ ID No.1 is the recognition site of Nde I, and the 7th-447th nucleotide of SEQ ID No.1 is The coding sequence of hemoglobin, the 450-455th nucleotide of SEQ ID No.1 is the recognition site of Xba I, and the 462-652th nucleotide of SEQ ID No.1 is the erythromycin resistance gene promoter , the 653-2134th nucleotide of SEQ ID No.1 is the coding sequenc...
Embodiment 2
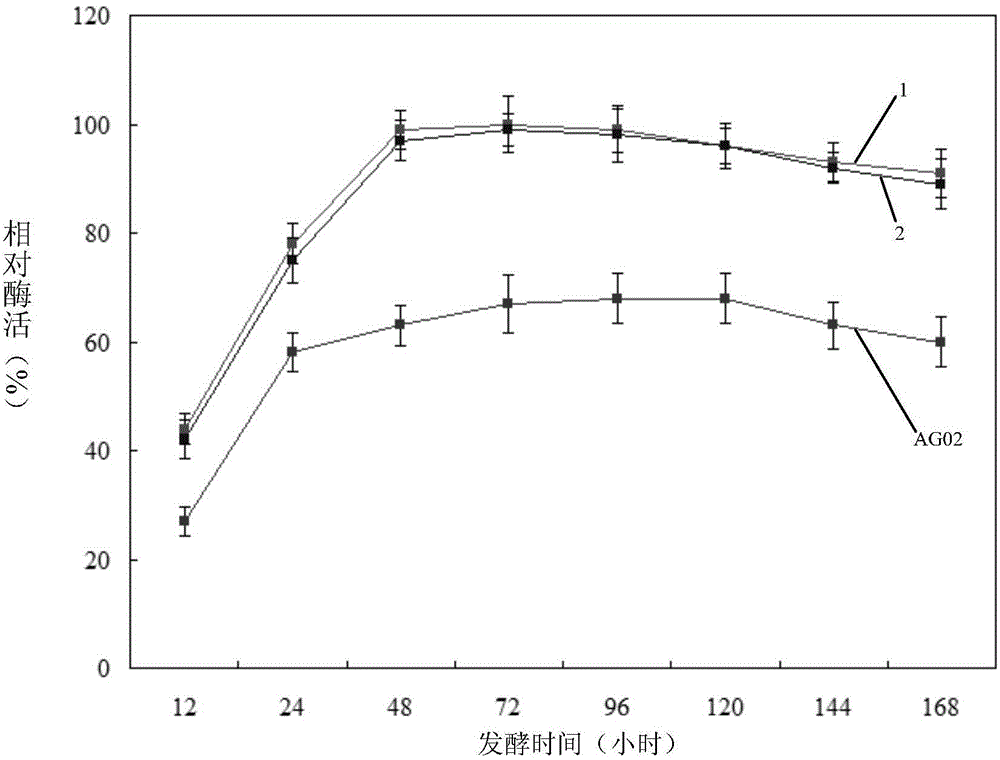
[0107] The cellulase activity of embodiment 2, AVG02
[0108] The experiment was repeated three times, and the results of each repeated experiment were as follows:
[0109] AVG02 spores (5×10 7 1) Add 50mL cellulase production medium (cellulase production medium consists of peptone, CMC-Na (sodium carboxymethylcellulose), NH 4 NO 3 、K 2 HPO 4 , NaCl and water, among which peptone, CMC-Na, NH 4 NO 3 、K 2 HPO 4 , NaCl mass percentages were 2%, 1%, 0.4%, 0.01%, 0.5%), cultured at 29°C and 220rpm for 20 hours to obtain AVG02 culture solution, and added 100mL cellulase to the AVG02 culture solution to produce culture Base, and diluted to OD 600nm was 0.1, and continued to cultivate at 29°C and 220rpm for 12 hours to obtain a 12-hour AVG02 fermentation broth. The 12-hour AVG02 fermentation broth was centrifuged at 14000 g for 10 minutes to obtain the 12-hour AVG02 fermentation supernatant. Determination of cellulase activity of 12-hour AVG02 fermentation supernatant (Mand...
Embodiment 3
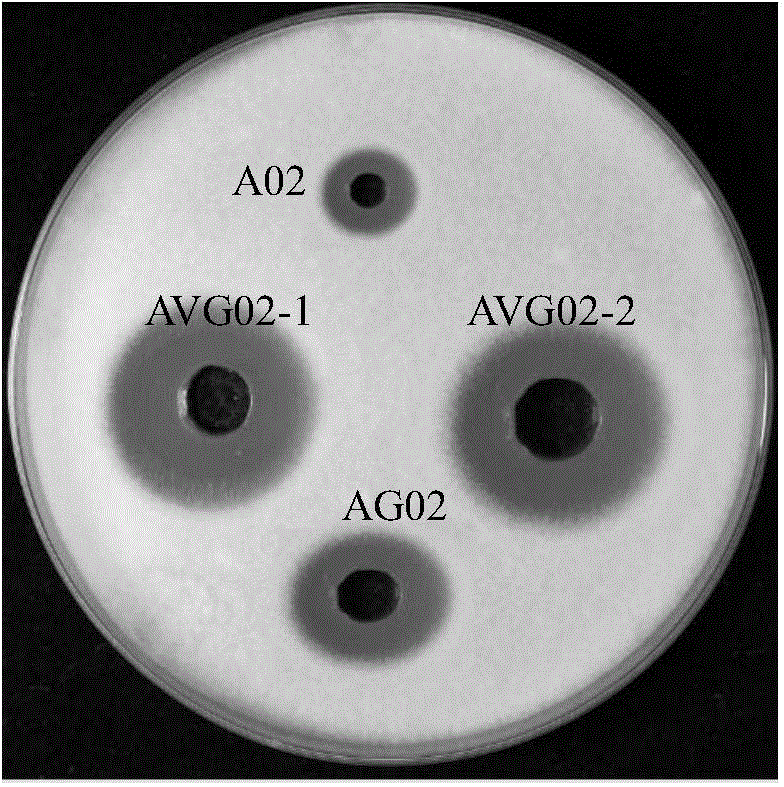
[0114] Embodiment 3, the ability of AVG02 to produce natamycin
[0115] One, the preparation of natamycin fermented liquid
[0116] The experiment was repeated three times, and the specific steps of each repeated experiment were as follows:
[0117] Inoculate the AVG02 in Example 1 onto Gao’s No. 1 slant medium, cultivate at 28°C for 7-10 days, and wait for it to produce a sufficient amount of spores, scrape the spores with a sterile platinum loop and inoculate 2-3 loops in 250ml Put the 50ml seed culture medium in the triangular flask on a temperature-controllable shaker, under the conditions of 28°C and 200rpm (rotation radius: 13mm), shake and cultivate at a constant temperature for 24h-30h; then inoculate it in 60 In the fermentation medium A (YSG medium) in the 500ml Erlenmeyer flask (the volume of each bottle is 100ml), the OD of each bottle after inoculation 600 The values were all 0.1; the inoculated shake flasks were shaken at 31°C for 0, 24, 48, 72, 96, and 120h ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com