Sustained-release nepafenac eye-drops preparation
A technology for nepafenac and ophthalmic preparations, applied in the field of ophthalmic pharmaceutical preparations, can solve problems such as not yet on the market, and achieve the effects of high intraocular bioavailability, strong penetration and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
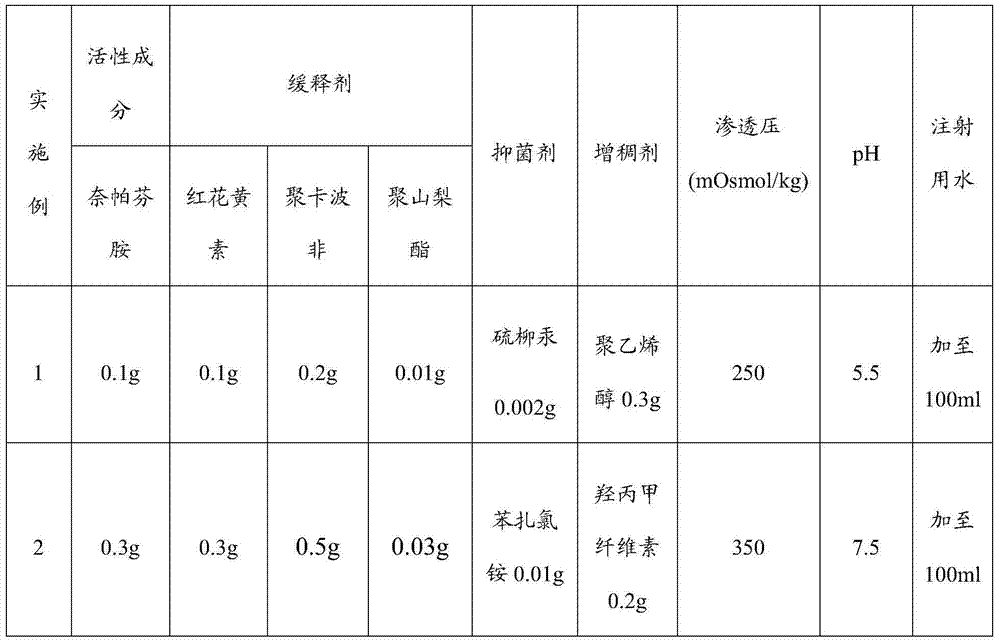
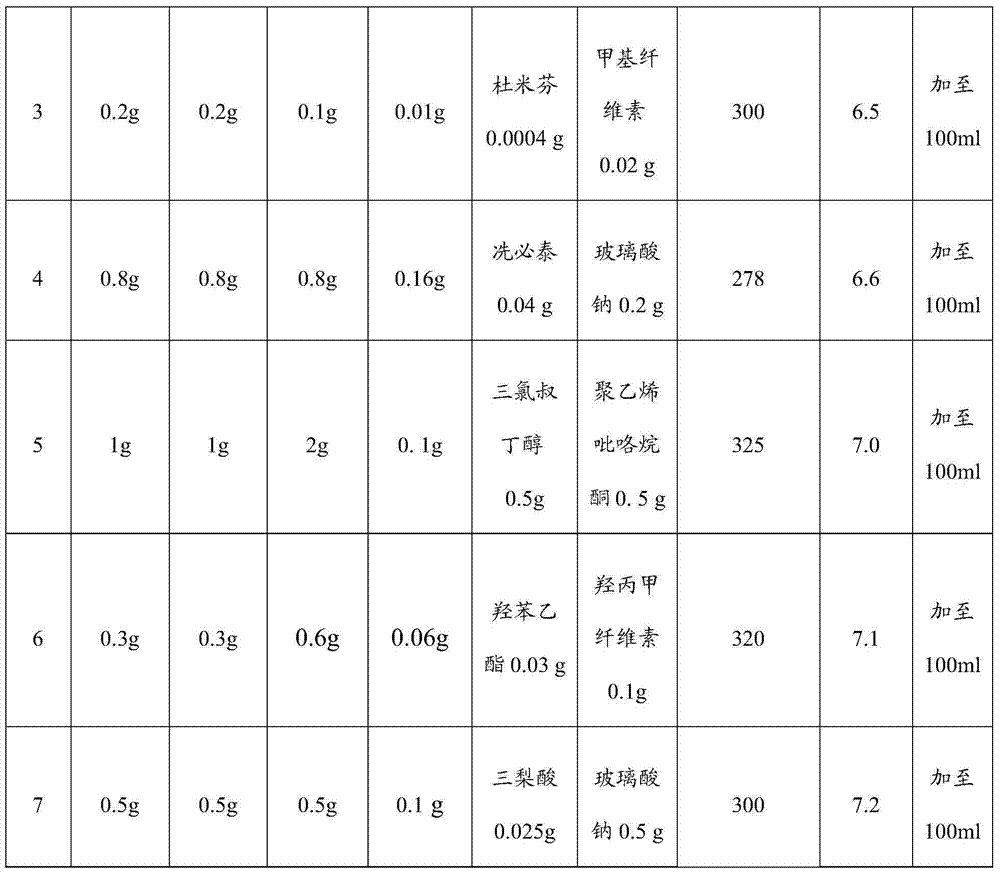
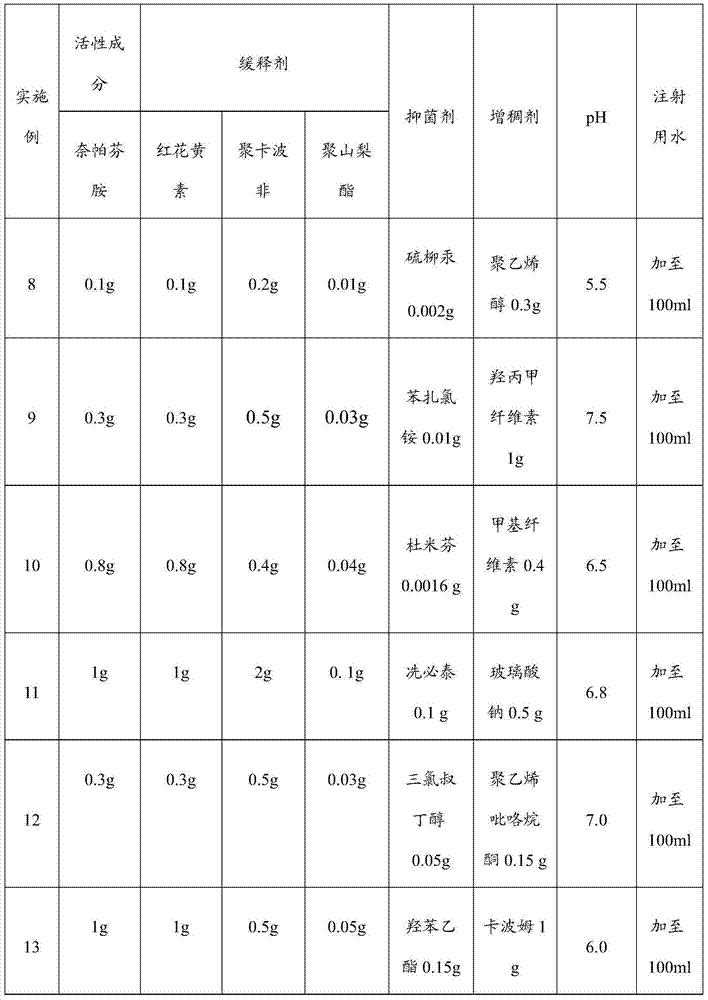
[0021] A sustained-release nepafenac ophthalmic preparation, comprising nepafenac, safflower flavin, polycarbophil, polysorbate, auxiliary materials and water for injection; the dosage form of the ophthalmic preparation is eye drops, Ophthalmic gel, ophthalmic ointment or any pharmaceutical dosage form suitable for topical ophthalmic application.
[0022] When the dosage form of the ophthalmic preparation is eye drops, the weight ratio is nepafenac: safflower flavin: polycarbophil: polysorbate: auxiliary material=1:1:0.5~2:0.05~0.2: 0.102-1.5, the mass fraction of nepafenac is 0.1%-1%, that is, the content of nepafenac is 0.1-1 part by weight per 100 parts by weight of the ophthalmic preparation.
[0023] The auxiliary material includes a bacteriostatic agent and a thickener, and the pH value of the eye drop is 5.5 to 7.5, and the osmolality of the eye drop is 250 to 350mOsmol / kg; the bacteriostatic agent is thimerosal, quaternary Any combination of one or more of ammonium sa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com