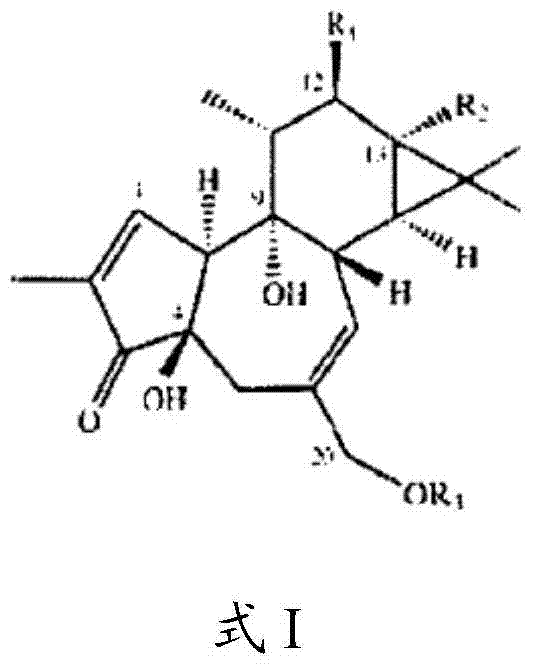
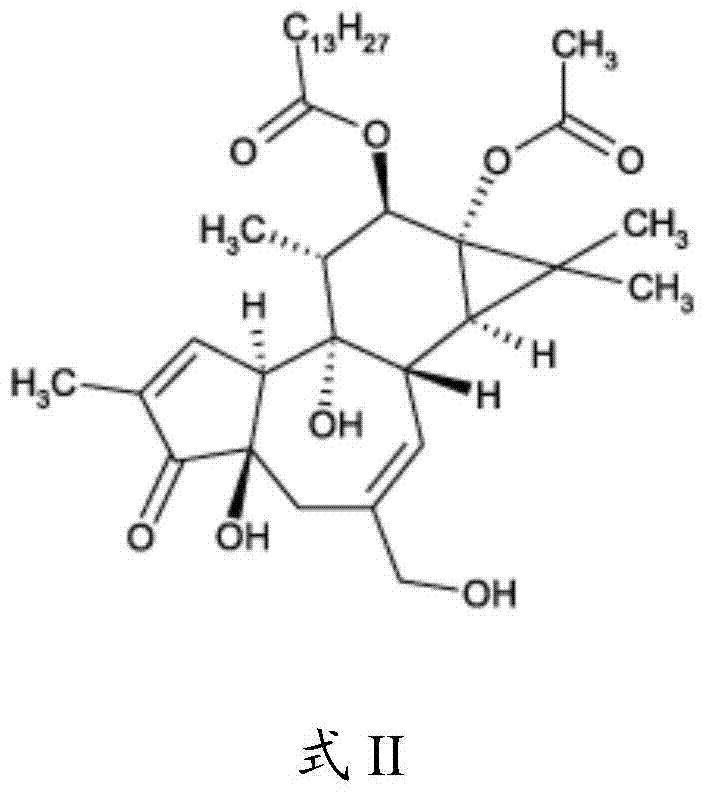
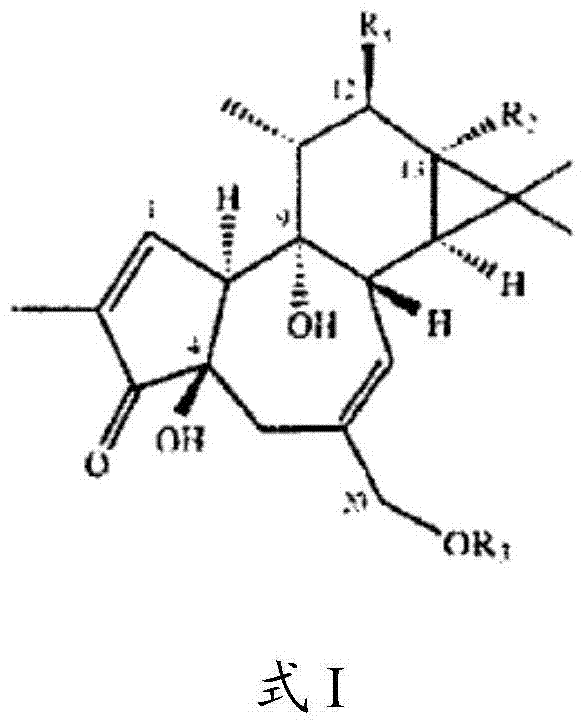
Compositions and methods of use of phorbol esters
A kind of phorbol ester, the technology of phorbol, applied in the composition of phorbol ester and use field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
[0247] The effect of TPA on peripheral white blood cells (WBC) and
[0248] Effect of hemoglobin (Hb) count:
[0249] Sarcoma 180 (S180) cells were injected into Kwen-Ming mice. On day three, mice were dosed with TPA intraperitoneally (i.p.) at 50, 100 or 200 μg / kg / day for 7 days. On the second day after completion of treatment, blood samples were obtained from the tails of treated mice for WBC and Hb analysis. WBC counts in the treatment groups (50, 100 or 200ug / kg / day for 7 days) were 16.1±7.4, 18.7±.3.0 and 20.7±.3.4×10 9 / L; the WBC count of the control group was 13.6±1.8×10 9 / L. The Hb in the treatment group was 136±11, 149±12 and 149±10 g / L, and the Hb in the control group was 134+-15 g / L. The results indicated that intraperitoneal injection of TPA could increase the peripheral WBC counts of mice in a dose-dependent manner, while the Hb levels of TPA-treated mice were not much affected compared with control mice.
Embodiment II
[0251] Dose Range Studies.
[0252] TPA is given to patients by intravenous (i.v.) infusion because of the strong local irritation it produces when administered. Inject the TPA solution in a sterile syringe into 200ml of sterile saline and mix well for intravenous infusion.
[0253] Toxicity and side effects of different TPA doses administered clinically:
[0254] (1) Administer TPA at 1 mg / patient / week:
[0255] Mix 1mg TPA in the solution with 200ml sterile saline for intravenous infusion, and the intravenous infusion is completed within 1 hour at a rate of 16μg / min. One hour after TPA administration, the patient started to have chills lasting about 30 minutes, followed by fever (the patient's body temperature reached 37.5-39.5°C, lasted 3-5 hours, and then returned to normal) with varying degrees of sweating. The above symptoms can be alleviated by giving patients glucocorticoids. TPA at this dose caused a few patients to bleed, some experienced short-term dyspnea, an...
Embodiment III
[0261] First clinical study in HIV+ patients treated with TPA
[0262] Twelve symptomatic patients (five males and seven females), aged 35 to 52 years, all acquired HIV via blood transfusion in 1995 and were HIV standard were treated with TPA Refractory to treatment. Each patient was administered a body weight-adjusted dose of TPA (75 μg / sq m) intravenously in 200 ml sterile saline over 1 hour. This dose is administered once daily for the first three days of treatment. This dose was then given to each patient every other day from day 4 to day 18, followed by a 6-month rest period, after which a second course of treatment followed the same protocol.
[0263] Blood samples were collected before the first dose of TPA and on days 4 and 40 of the treatment cycle. CD3, CD4 and CD8 levels in peripheral blood were measured using monoclonal antibodies (Becton Dickson Scientific Co., Franklin Lakes, NJ) and flow cytometry (B.D. Bioscience, San Diego, CA).
[0264] As can be seen i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com