Humanized tau antibody
A technology of humanized antibodies and antibodies, applied in chemical instruments and methods, anti-animal/human immunoglobulins, instruments, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
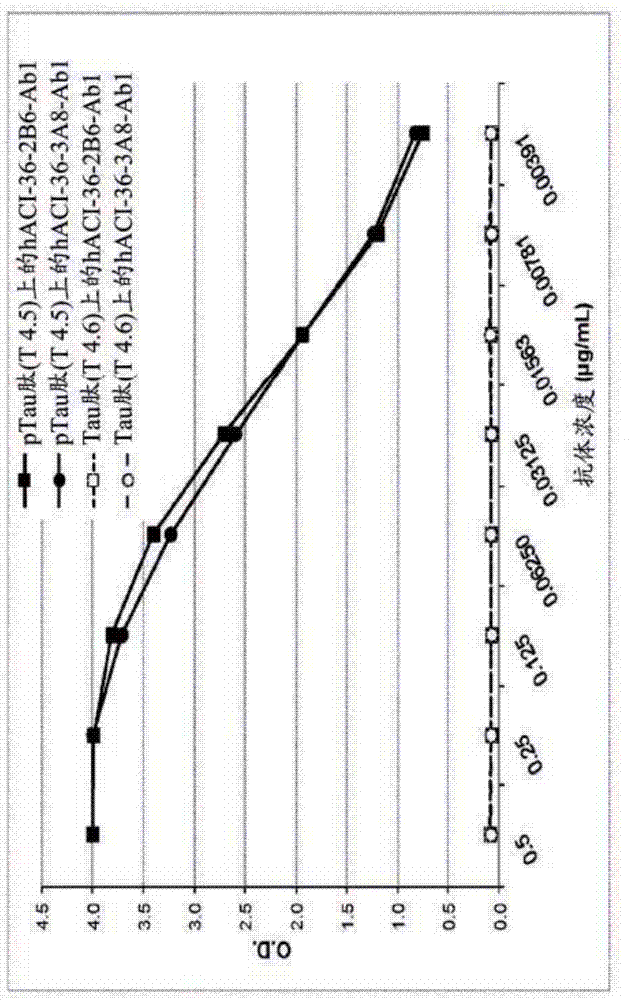
[0363] Example 1. Binding of hACI-36-2B6-Ab1 and hACI-36-3A8-Ab1 to T4 peptide detected by ELISA
[0364] 1.1. method
[0365] 1.1.1 Phosphorylated Tau binding assay
[0366] Antibody binding to pTau was tested using an ELISA assay. Nunc MaxiSorp 96-well plates (Nunc, Denmark) were coated with 10 μg / mL of Tau-derived peptides Tau401 to 418 (phosphorylated (T4.5) or unphosphorylated (T4.6) at serine at position 409). Coatings were left overnight at 4°C in phosphate buffered saline (PBS; Sigma-Aldrich, Switzerland). Plates were washed extensively with 0.05% Tween20 / PBS and then blocked with 1% bovine serum albumin (BSA; Sigma-Aldrich) in 0.05% Tween20 / PBS for 1 hour at 37°C. Antibody-containing supernatants to be tested were then added in 8 two-fold dilutions (starting at 0.5 μg / mL) and incubated at 37°C for 2 hours. Plates were then washed as previously described, and alkaline phosphatase (AP)-conjugated goat anti-human IgG (Jackson ImmunoResearch Laboratories, England)...
Embodiment 2
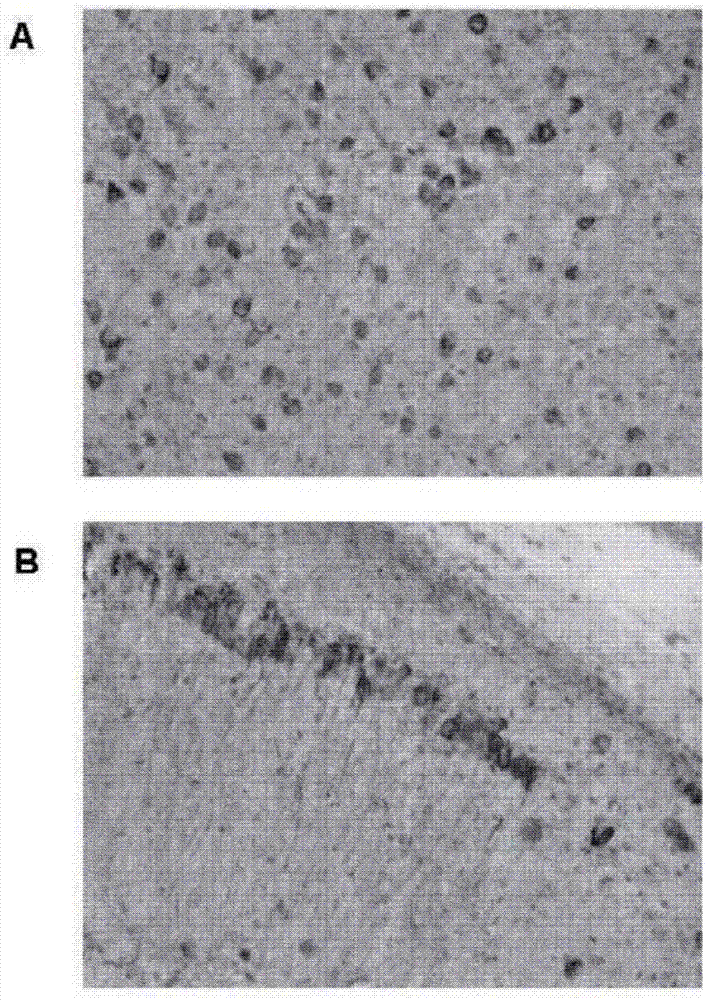
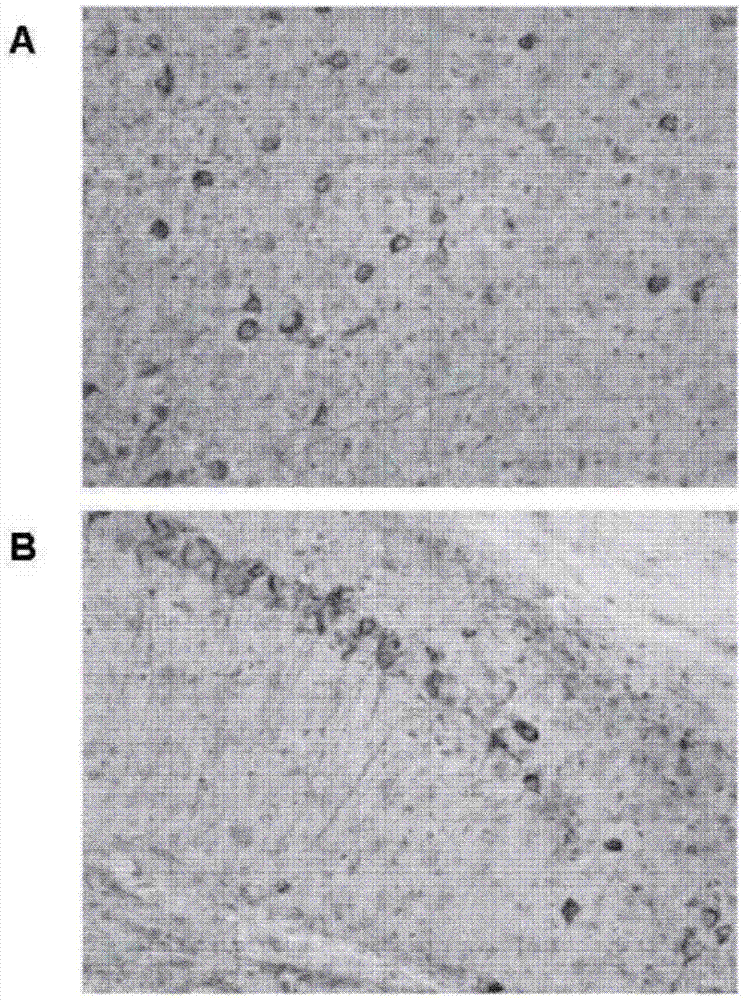
[0369] Example 2. Staining of pTau in the brains of 20-month-old transgenic tauopathic (biGT) mice using hACI-36-2B6-Ab1 and hACI-36-3A8-Ab1 by TAUPIR
[0370] 2.1. method
[0371] 2.1.1 Binding of anti-Tau antibody to Tau tangles on brain slices from Tau transgenic animals (TAUPIR)
[0372] Small tau lesions using aged (>18 months old) bigenic transgenic biGT (GSK-3β transgenic mice crossed with TPLH mice containing the longest isoform (441 aa) of human Tau with the P301L mutation) Rat brain slices. Brain slices were washed in PBS for 5 min and then incubated in 1.5% HO in PBS:MeOH (1:1) at ambient temperature. 2 o 2 Incubate for 15 min to block endogenous peroxidase activity. After sections were washed 3 times in PBST (PBS / 0.1% TritonX100), they were incubated in PBST+10% FCS (fetal calf serum) blocking solution for 30 min at room temperature. Sections were then incubated overnight at 4°C with undiluted supernatant containing the antibody to be tested. Sections wer...
Embodiment 3
[0375] Example 3. Binding Study II: Detection of Antibody Affinity by Biacore / SPR
[0376] 3.1 Method
[0377] 3.1.1 SPR binding assay
[0378] To assess the binding interaction between hACI-36-2B6-Ab1 and peptide T4.5, the antibody hACI-36-2B6-Ab1 was immobilized on the sensor chip, and T4.5 was then injected as an analyte.
[0379] SPR experiments were performed on a Biacore T100 instrument (GE Healthcare). Reagents for immobilization (EDC, NHS and ethanolamine) and sensor chip CM7 (carboxymethyl dextran) were purchased from GE Healthcare. The running buffer was PBS (Dulbecco's PBS, Sigma). To properly orient the antibody for binding to peptide T4.5, the antibody was coupled to the sensor surface via protein G. For this, recombinant protein G (Sigma) was diluted to 50 μg / mL from a stock solution (2 mg / mL) in water with 10 mM sodium acetate, pH 4.0 (GE Healthcare). This protein solution was then coupled to the flow cell (fc)2 of a CM7 sensor chip pre-activated with EDC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com