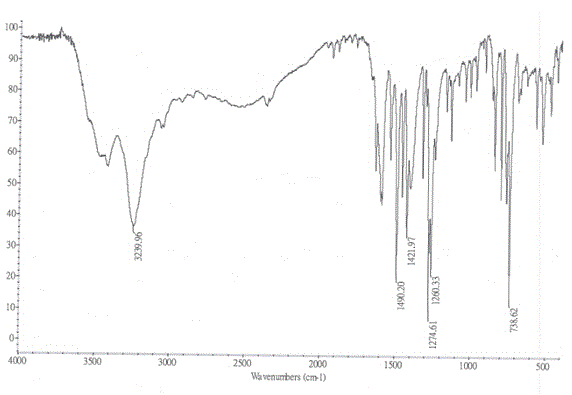
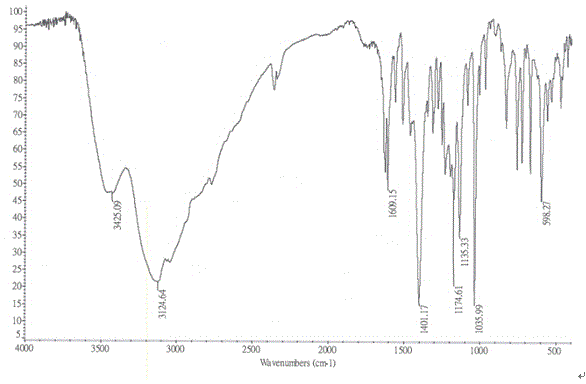
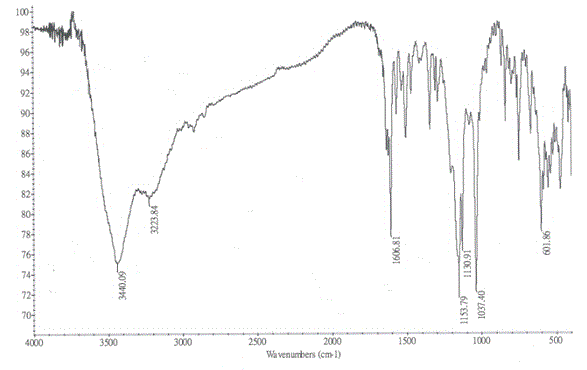
Method for improving water solubility of hydroxybenzimidazolyl compound
A technology of hydroxybenzimidazole and compound, which is applied in the field of drug synthesis, and can solve the problems of low solubility, poor water solubility and limited application of hydroxyphenylbenzimidazole compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Take 1.0 g (8.19 mmol) of salicylaldehyde and 0.85 g (8.19 mmol) of sodium sulfonate, and dissolve them in 25 mL of 95% ethanol to form a solution. At room temperature, it was placed in a 50 mL round bottom flask and reacted for 4 h under magnetic stirring to form a precipitate. Then add 0.89 g (8.19 mmol) of o-phenylenediamine and 25 mL of DMF into the round bottom flask, heat and reflux for 2 h, pour 10 times the amount of water into the solution, suction filter, dry the obtained solid, and recrystallize with ethanol , to 1.55g, yield 90%.
[0041] Dissolve 1.55 g (7.37 mmol) of 2-(2-hydroxyphenyl)benzimidazole in 10 g of concentrated sulfuric acid, heat it in a water bath for 2 h, inject 10 times the amount of water into the reactant, and let it stand for a while A sulfonic acid is then precipitated. First neutralize the sulfonation solution with an appropriate amount of calcium bicarbonate to convert the product into a water-soluble sodium salt, and filter out wat...
Embodiment 2
[0047] Take 1.0 g (8.19 mmol) of salicylaldehyde and 0.94 g (9.00 mmol) of sodium sulfonate, and dissolve them in 25 mL of 95% ethanol to form a solution. At room temperature, it was placed in a 50 mL round bottom flask and reacted for 4 h under magnetic stirring to form a precipitate. Then add 0.97 g (9.0 mmol) o-phenylenediamine and 25 mL DMF to the round bottom flask, heat to reflux for 2 h, pour 10 times the amount of water into the solution, suction filter, dry the obtained solid, and recrystallize with ethanol, 1.53 g was obtained with a yield of 89%.
[0048] Dissolve 1.53 g (7.29 mmol) of 2-(2-hydroxyphenyl)benzimidazole in 10 g of concentrated sulfuric acid, heat it in a water bath for 2 h, inject 10 times the amount of water into the reactant, and let it stand for a while A sulfonic acid precipitated. First neutralize the sulfonation solution with an appropriate amount of calcium bicarbonate to convert the product into a water-soluble sodium salt, and filter out wa...
Embodiment 3
[0050] Take 1.0 g (8.19 mmol) of salicylaldehyde and 1.02 g (9.83 mmol) of sodium sulfonate, and dissolve them in 25 mL of 95% ethanol to form a solution. At room temperature, it was placed in a 50 mL round bottom flask and reacted for 4 h under magnetic stirring to form a precipitate. Then add 1.06 g (9.83 mmol) o-phenylenediamine and 25 mL DMF to the round bottom flask, heat to reflux for 2 h, pour 10 times the amount of water into the solution, suction filter, dry the obtained solid, and recrystallize with ethanol , the yield was 91%.
[0051] Dissolve 1.57 g (7.45 mmol) of 2-(2-hydroxyphenyl)benzimidazole in 10 g of concentrated sulfuric acid, heat it in a water bath for 2 h, inject 10 times the amount of water into the reactant, and let it stand for a while A sulfonic acid precipitated. First neutralize the sulfonation solution with an appropriate amount of calcium bicarbonate to convert the product into a water-soluble sodium salt, and filter out water-insoluble impuri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com