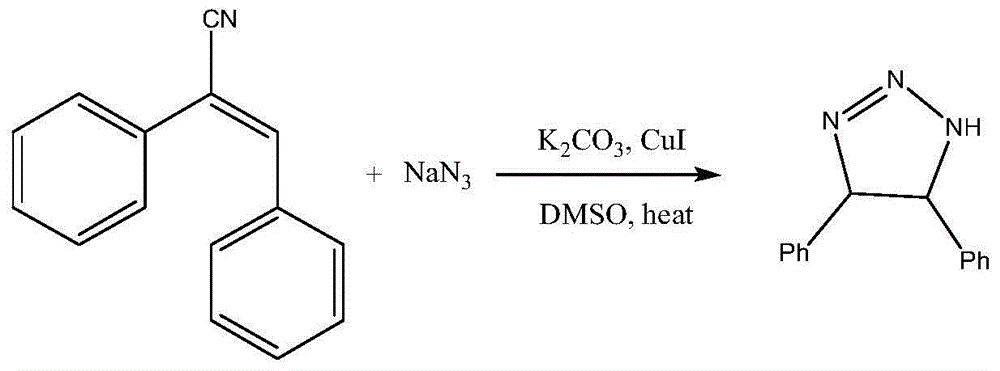
Method for synthesizing 4,5-diphenyl-1,4,5-trihydro-1,2,3-triazole
A synthesis method and a diphenyl technology, which are applied in the field of synthesis of 4,5-diphenyl-1,4,5-trihydro-1,2,3-triazole, can solve problems such as lack, and achieve Moderate polarity, high purity, good effect of separating and purifying products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] General reaction formula of the present invention is:
[0015]
[0016] The present invention provides a synthesis method of 4,5-diphenyl-1,4,5-trihydro-1,2,3-triazole, adding 1mmol 1,2-diazole to a 50ml round bottom flask successively Phenylacrylonitrile, 2mmol potassium carbonate, 2mmol sodium azide, 0.2mmol cuprous iodide and 10ml DMSO, heated to 100°C for reaction, after 3h the reaction was completed, cooled, the system was poured into 100ml water, the mixture was washed with ethyl acetate The ester was extracted 2-3 times, and the solvent was evaporated after merging the organic layers, and the residue was subjected to thin chromatography, and the column chromatography adopted a silica gel stationary phase, and the eluent was a mixture of ethyl acetate and cyclohexane, and the ethyl acetate and The mixing volume ratio of cyclohexane is 1:4. 4,5-Diphenyl-1,4,5-trihydro-1,2,3-triazole was isolated.
[0017] The molar ratio of the 1,2-diphenylacrylonitrile, potas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com