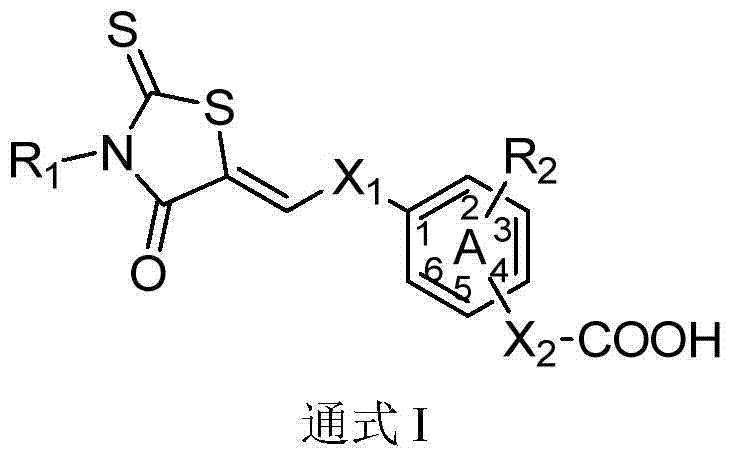
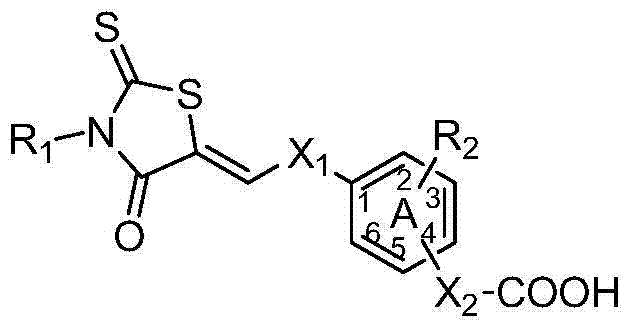
3,5-disubstituted rhodanine anti-apoptotic protein bcl-2 inhibitor and its preparation method and application
An anti-apoptotic protein, rhodanine technology, applied in the field of medicine, can solve the problems of drug resistance, poor selectivity, and limited clinical application of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
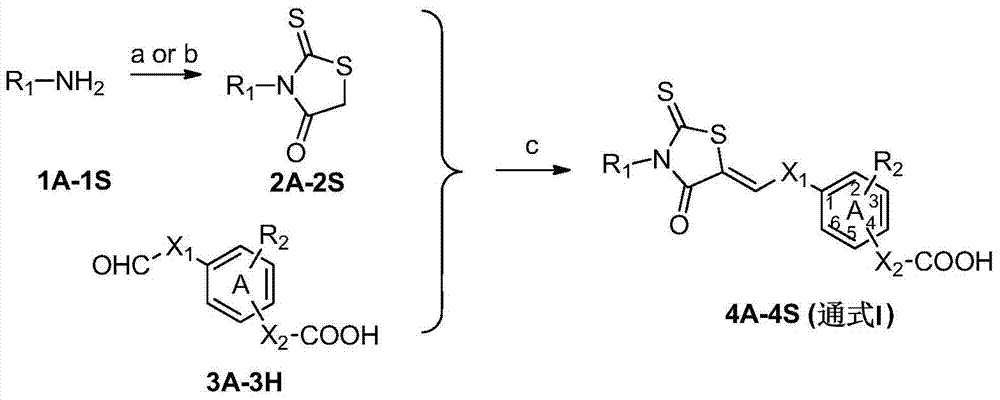
[0105] Example 1. (Z)-4-[[4-[(3-phenyl-2-thio-4-thiazolidinedione-5-ylidene)methyl]phenoxy]methyl]benzoic acid Synthesis of (4AA)
[0106] Synthesis of 3-phenyl-2-thioxo-4-thiazolidinedione (2A)
[0107] Under ice-bath conditions, add 2mL of ethanol to aniline (1A, 0.93g, 10mmol) and triethylamine (5.05g, 50mmol), stir for 10 minutes, add carbon disulfide (1.52g, 20mmol) dropwise, and a light yellow solid precipitates out of the system. Filter and wash with ether. The obtained solid was added to 5 mL aqueous solution of sodium chloroacetate (1.28 g, 11 mmol), stirred until the solid was dissolved, added hydrochloric acid (6 mol / L, 14 mL), stirred at 85° C. for 0.5 hour, cooled, and filtered to obtain a pale yellow solid, which was washed with Chloroform recrystallization gave a pale yellow solid (1.60 g, 80%). M.p.: 195-197°C. 1 H NMR (600MHz, CDCl 3 )δ7.54 (td, J = 7.2Hz, 1.8Hz, 2H), 7.50 (tt, J = 7.2Hz, 1.8Hz, 1H), 7.20 (dt, J = 7.2Hz, 1.8Hz, 2H), 4.20 ( s,2H).
[010...
Embodiment 2
[0111] Example 2. (E)-3-[4-[[4-[(Z)-(3-phenyl-2-thio-4-thiazolidinedione-5-ylidene)methyl]phenoxy Synthesis of yl]methyl]phenyl]acrylic acid (4AB)
[0112] The preparation methods of intermediates and target compounds are as in Example 1. Yield 62%; m.p.>250°C; 1 H NMR (300MHz, DMSO-d 6 )δ12.42(s,1H),7.82(s,1H),7.73(d,J=8.1Hz,2H),7.68(d,J=9.0Hz,2H),7.55(m,6H),7.41( m,2H),7.23(d,J=9.0Hz,2H),6.55(d,J=16.2Hz,1H),5.27(s,2H).HRMS(AP-ESI)m / z calcd for C 26 h 19 NO 4 S 2 [M-H] - 472.0683,found 472.0663.
Embodiment 3
[0113] Example 3. (Z)-4-nitro-3-[[4-[(3-phenyl-2-thio-4-thiazolidinedione-5-ylidene)methyl]phenoxy] Synthesis of methyl]benzoic acid (4AC)
[0114] The preparation methods of intermediates and target compounds are as in Example 1. Yield 84%; m.p.229-230°C; 1 H NMR (400MHz, DMSO-d 6 )δ13.68(s,1H),8.31(d,J=1.6Hz,1H),8.25(d,J=8.4Hz,1H),8.14(dd,J=8.4Hz,1.6Hz,1H),7.83 (s,1H),7.70(d,J=8.8Hz,2H),7.58-7.52(m,3H),7.43-7.41(m,2H),7.26(d,J=8.8Hz,2H),5.62( s,2H).HRMS(AP-ESI)m / z calcd for C 24 h 16 N 2 o 6 S2 [M+H] + 493.0523,found 493.0531.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com