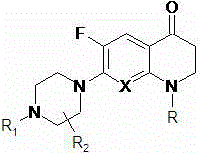
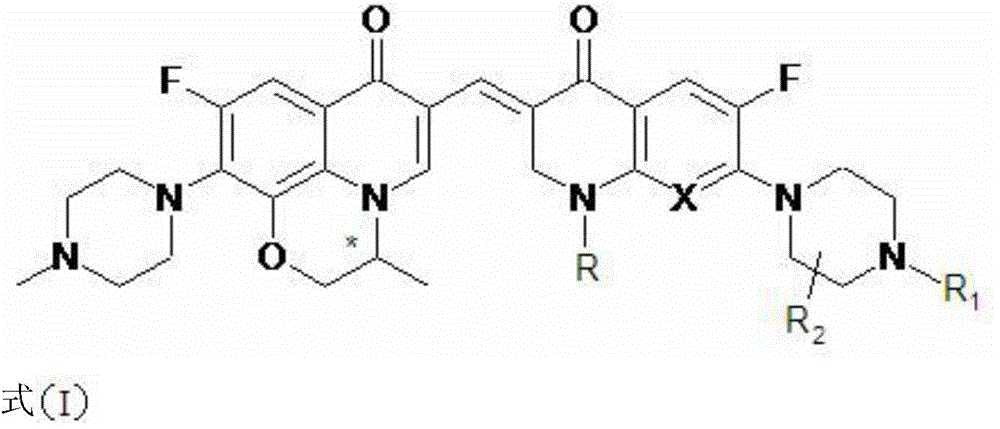
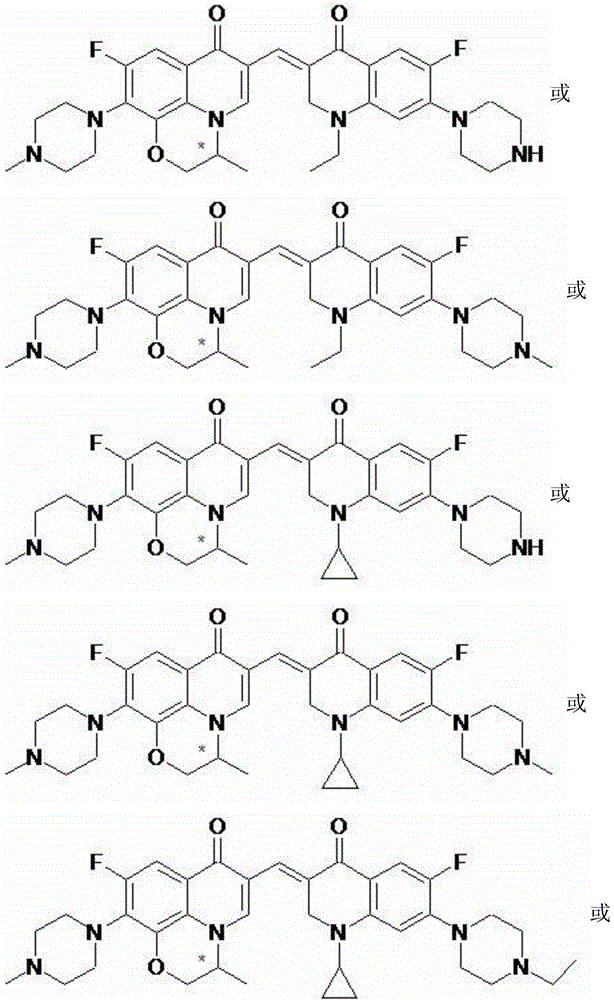
A kind of 3,3'-methylene-bisfluoroquinolone derivative of chiral oxazinoquinoline ring and its preparation method and application
A technology of bisfluoroquinolone and quinoline ring, applied in the field of drug synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (S)-6-fluoro-7-(4-methyl-piperazin-1-yl)-1,8-(3,1-oxopropyl)-3-[1-ethyl-6-fluoro- 7-piperazin-1-yl-2,3-dihydro-quinolin-4(1H)-one-3-ylidenemethyl]-quinolin-4(1H)-one (I-1), its chemical The structural formula is:
[0036]
[0037] That is, R in formula I is an ethyl group, and R 1 and R 2 is a hydrogen atom, and X is a hydrocarbon group.
[0038] The preparation method of the compound is: take 6-fluoro-7-(4-methyl-piperazin-1-yl)-1,8-(3,1-oxopropyl)-quinoline-4(1H)- Ketone (VI) 0.55g (1.6mmol) and 1-ethyl-6-fluoro-7-piperazin-1-yl-2,3-dihydro-quinolin-4(1H)-one (III-1) 0.44g (1.6mmol) was dissolved in 20mL of absolute ethanol, 0.2mL of piperidine was added dropwise, and the reaction was refluxed for 24h. After standing for 12 hours, the resulting solid was collected by filtration and recrystallized from DMF-ethanol (V:V=3:1) to obtain a light yellow crystal (I-1), with a yield of 82%, m.p.228-232°C. 1 H NMR (400MHz, DMSO-d 6 )δ: 7.91~7.88(brs,2H,2-H,3-CH=),7.7...
Embodiment 2
[0040] (S)-6-fluoro-7-(4-methyl-piperazin-1-yl)-1,8-(3,1-oxopropyl)-3-[1-ethyl-6-fluoro- 7-(4-methylpiperazin-1-yl)-2,3-dihydro-quinolin-4(1H)-one-3-ylidenemethyl]-quinolin-4(1H)-one (I -2), its chemical structural formula is:
[0041]
[0042] That is, R in formula I is an ethyl group, and R 1 is methyl, R 2 is a hydrogen atom, and X is CH.
[0043]The preparation method of the compound is: take 6-fluoro-7-(4-methyl-piperazin-1-yl)-1,8-(3,1-oxopropyl)-quinoline-4(1H)- Ketone (VI) 0.55g (1.6mmol) and 1-ethyl-6-fluoro-7-(4-methylpiperazin-1-yl)-2,3-dihydro-quinoline-4(1H)- Ketone (III-2) 0.50g (1.7mmol) was dissolved in 20mL absolute ethanol, triethylamine 0.2mL was added dropwise, and the reaction was refluxed for 20h. After standing for 12 hours, the resulting solid was collected by filtration and recrystallized with DMF-ethanol (V:V=3:1) to obtain a light yellow crystal (I-2), with a yield of 85%, m.p.225-227°C. 1 H NMR (400MHz, DMSO-d 6 )δ: 7.90~7.87(brs,2H,2-H,3-...
Embodiment 3
[0045] (S)-6-fluoro-7-(4-methyl-piperazin-1-yl)-1,8-(3,1-oxopropyl)-3-[1-cyclopropyl-6-fluoro -7-piperazin-1-yl-2,3-dihydro-quinolin-4(1H)-one-3-ylidenemethyl]-quinolin-4(1H)-one (I-3), which The chemical structural formula is:
[0046]
[0047] That is, R in formula I is cyclopropyl, R 1 and R 2 is a hydrogen atom, and X is a hydrocarbon group.
[0048] The preparation method of the compound is: take 6-fluoro-7-(4-methyl-piperazin-1-yl)-1,8-(3,1-oxopropyl)-quinoline-4(1H)- Ketone (VI) 0.55g (1.6mmol) and 1-cyclopropyl-6-fluoro-7-piperazin-1-yl-2,3-dihydro-quinolin-4(1H)-one (III-3 ) 0.52g (1.8mmol) was dissolved in 20mL absolute ethanol, piperidine 0.2mL was added dropwise, and the reaction was refluxed for 22h. After standing for 24 hours, the resulting solid was collected by filtration and recrystallized from DMF-ethanol (V:V=5:1) to obtain a light yellow crystal (I-3), with a yield of 86%, m.p.232-234°C. 1 H NMR (400MHz, DMSO-d 6 )δ: 7.92~7.88(brs,2H,2-H,3-CH=),7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com