Fluorescent probe targeting MUC1 for in-vivo ovarian cancer tissue and preparation method of fluorescent probe
A fluorescent probe, ovarian cancer technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., to achieve the effect of increased expression and strong penetration ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
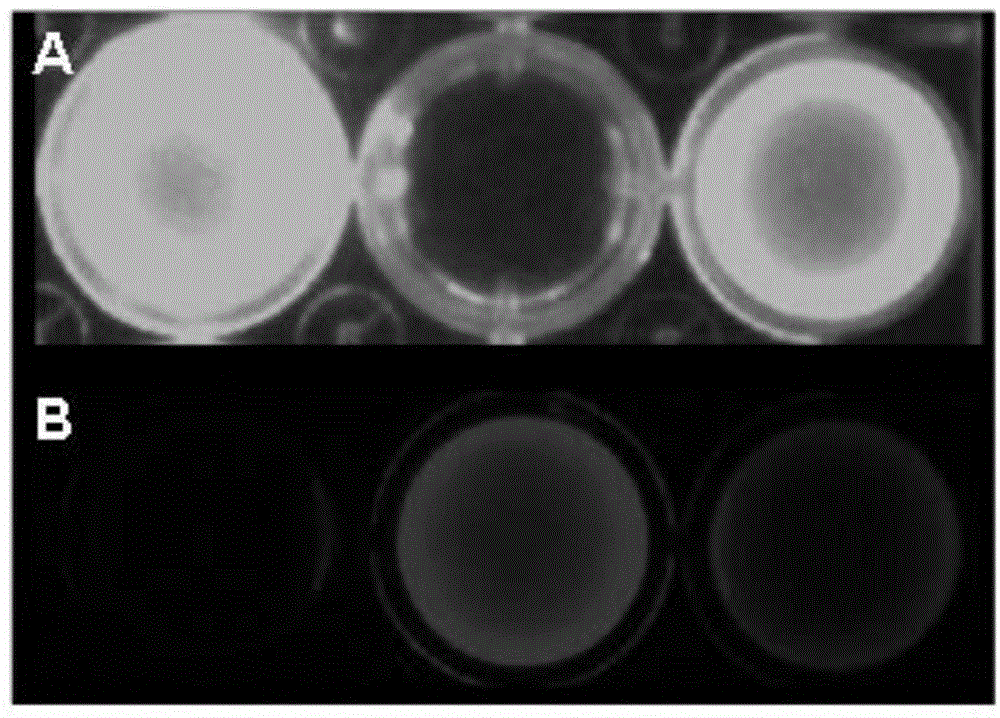
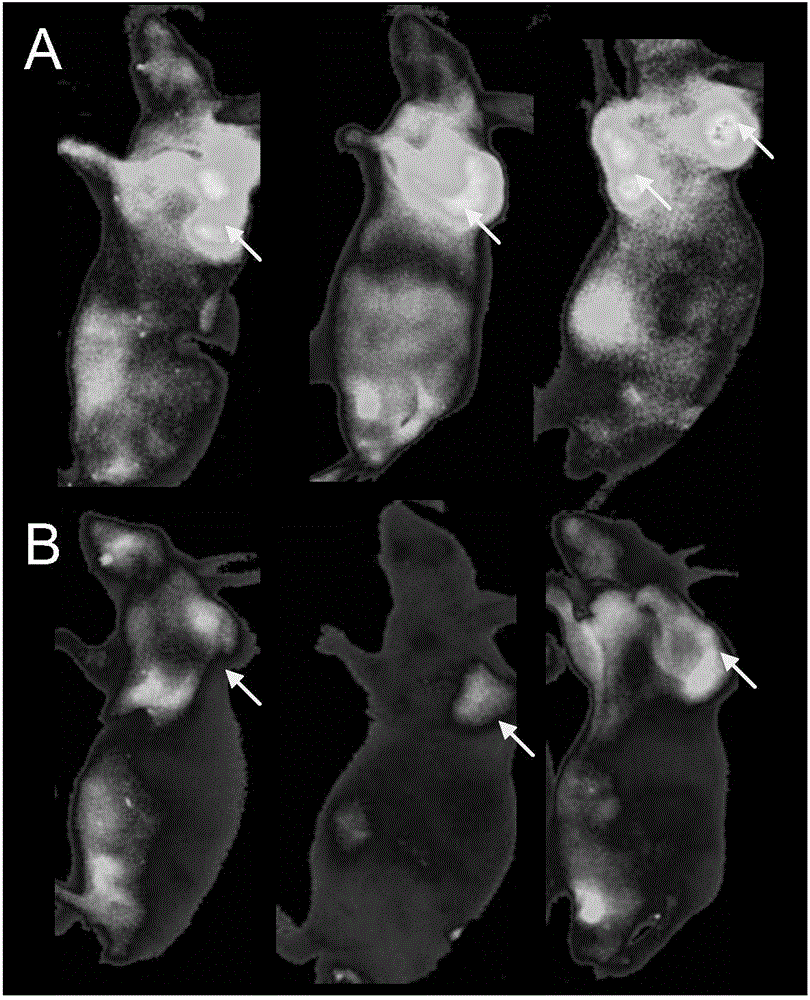
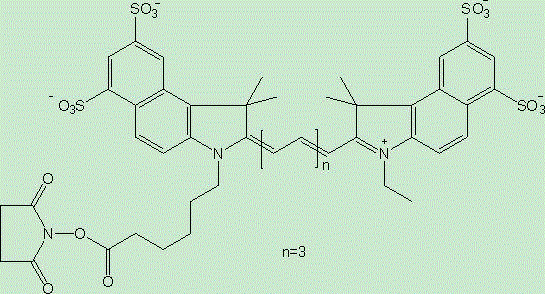
[0022] Example 1: Dilute the anti-MUC1 antibody with 0.1M sodium bicarbonate buffer with a pH value of 8.5 to a final concentration of 1 mg / ml, and then mix the anti-MUC1 antibody / fluorescent dye / Cy5.5 in a ratio of 1:1:1 Labeling was carried out by reacting at 4oC in the dark for 2 hours, and the reactants were filtered and chromatographed through a desalting column (Zeba®, Thermo Scientific, USA) to remove unreacted dyes to obtain the fluorescent probe targeting MUC1 of the living ovarian cancer tissue , by detecting the antibody and bound dye content in a UV spectrophotometer as 1 / 0.7 / 0.8.
example 2
[0023] Example 2: Dilute the anti-MUC1 antibody with 0.1M sodium bicarbonate buffer with a pH value of 8.5 to a final concentration of 1 mg / ml, then anti-MUC1 antibody / fluorescent dye / Cy5.5 at a ratio of 1:10:10 at 4oC After 2 hours of dark reaction for labeling, the reactant was filtered and chromatographed through a desalting column to remove unreacted dye to obtain the fluorescent probe targeting MUC1 of the living ovarian cancer tissue, and detect the antibody and The combined dye content is 1 / 2.5 / 2.8.
example 3
[0024] Example 3: Dilute the anti-MUC1 antibody with 0.1M sodium bicarbonate buffer with a pH value of 8.5 to a final concentration of 1 mg / ml, then anti-MUC1 antibody / fluorescent dye / Cy5.5 at 4oC at a ratio of 1:20:20 After 2 hours of dark reaction for labeling, the reactant was filtered and chromatographed through a desalting column to remove unreacted dye to obtain the fluorescent probe targeting MUC1 of the living ovarian cancer tissue, and detect the antibody and The combined dye content is 1 / 1.9 / 2.0.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com