Quinone compounds and their uses for the treatment of cancer
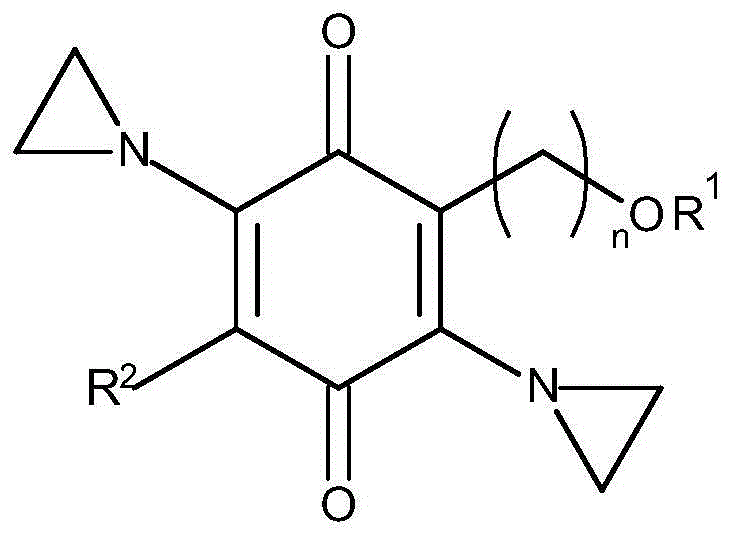
A compound, cancer technology, applied in the field of 2,5-diaziridinylbenzoquinone compound, prodrug of cancer cells, cancer treatment, can solve problems such as poor solubility, hindering effective therapeutic preparations, increased cytotoxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
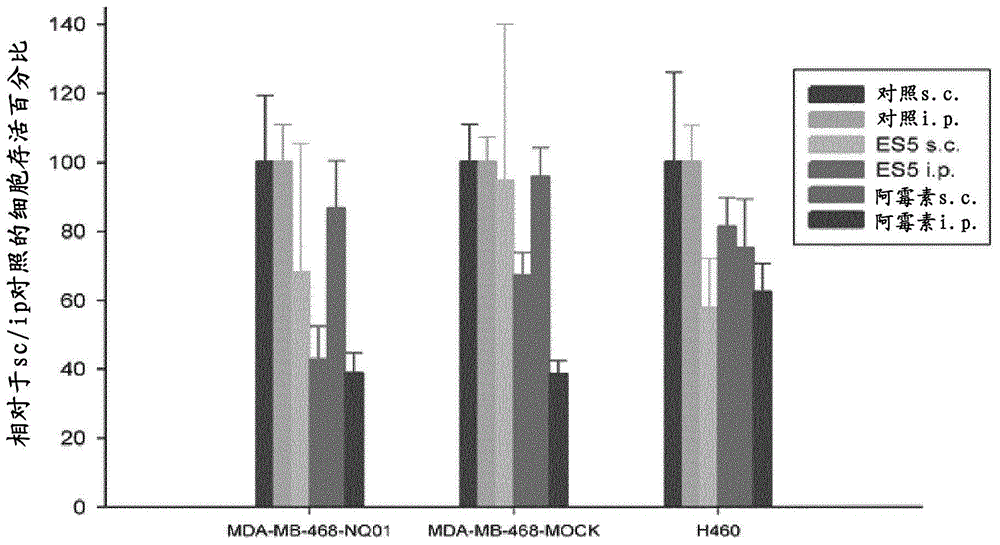
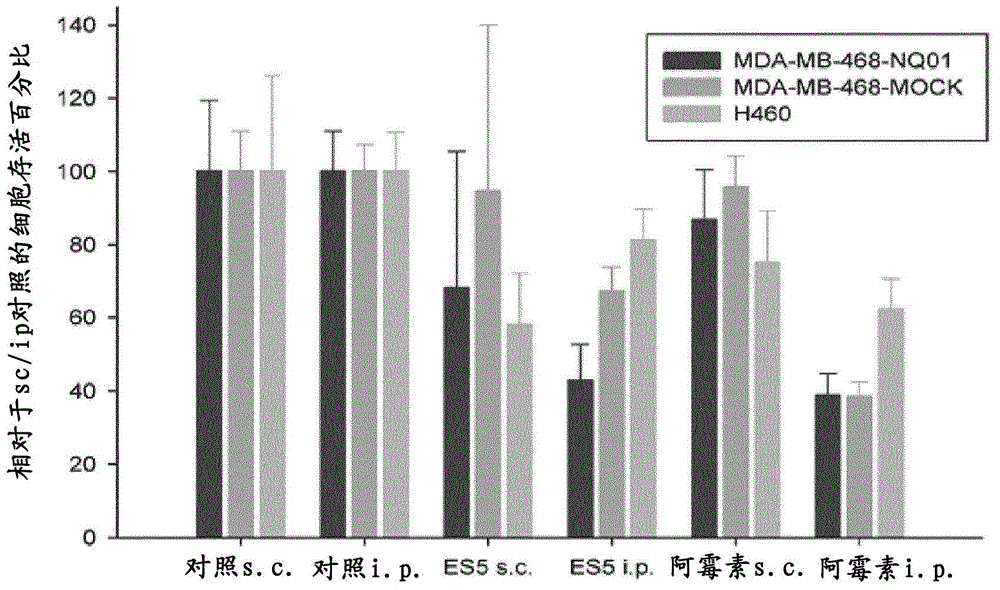
Image
Examples
Embodiment
[0085] Materials and methods
[0086] Synthesis of Es5
[0087]
[0088] 1,4-Dimethoxy-2-methyl-benzene 2[1]
[0089]
[0090] Will be dissolved in diethyl ether (90cm 3 ) of 2,5-dimethoxytoluene 1 (9.0g, 59.21mmol) was dissolved in chloroform (30cm 3 ) in a stirred solution of iodine monochloride (10.17g, 62.65mmol). After adding 10% sodium thiosulfate (150cm 3 ), the mixture was stirred overnight. at 75cm 3 The organics were extracted twice with diethyl ether. Combine organics to saturate with NaHCO 3 Aqueous solution (150cm 3 ), salt water (100cm 3 ) washed, dried (MgSO 4 ) and dried under reduced pressure. The final solid was recrystallized from methanol to give the title compound 2 as a red solid (11.3 g, 69%).
[0091] R f 0.52 (SiO 2 , Hex 3:1 EtOAc).
[0092] Mp 80-82°C (literature 81-82°C (Reed et al., JACS, 120(38):9729-9734, 1998).
[0093] 1 H NMR (400MHz, CDCl 3 )2.12 (3H,s,CH 3 ),3.72(3H,s,OCH 3 ),3.75(3H,s,OCH 3 ), 6.60 (1H, s, ArH)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| composition ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com