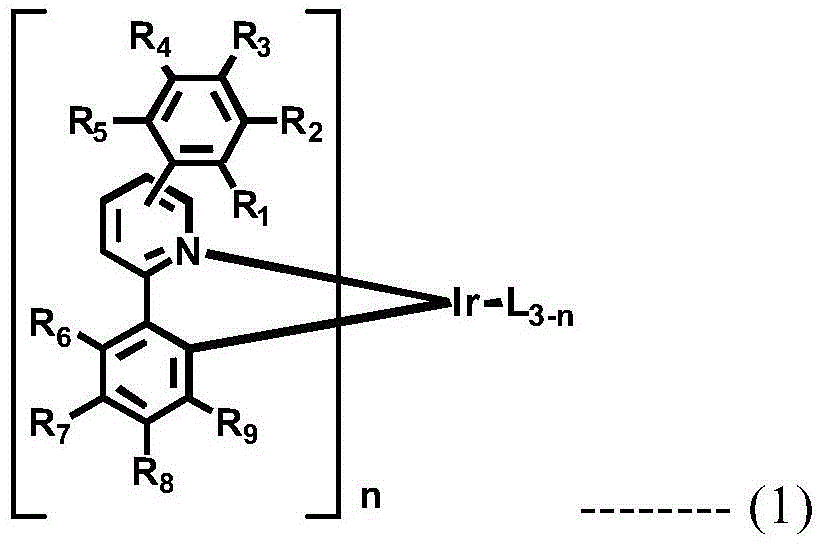
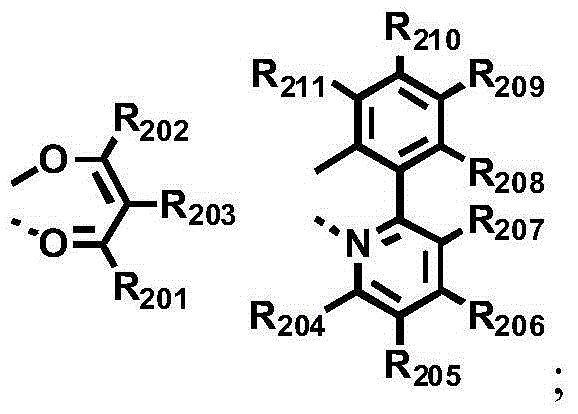
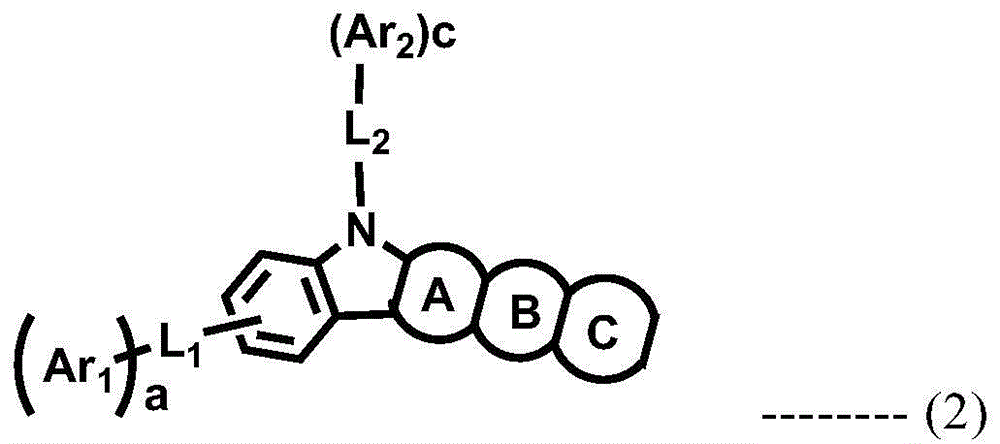
A novel combination of a host compound and a dopant compound and an organic electroluminescence device comprising the same
A compound and dopant technology, applied in electroluminescent light sources, electro-solid devices, indium organic compounds, etc., can solve the problems of undisclosed luminescent materials emitting yellow-green light, etc., and achieve the goal of improving power efficiency and working life. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Embodiment 1: the preparation of compound D-1
[0069]
[0070] Preparation of compound 1-1
[0071] 5g (34mmol) of 2,4-dichloropyridine, 16g (135mmol) of phenylboronic acid, Pd(PPh 3 ) 4 3.9g (2.4mmol), K 2 CO 3 23g (135mmol), 100mL toluene, 50mL ethanol and 50mL H 2 After O was added to the flask, the mixture was stirred at 120 °C for 6 hours. Then, the reaction mixture was dried and separated by column to obtain Compound 1-16.4 g (82%).
[0072] Preparation of compound 1-2
[0073] Compound 1-14g (17mmol), IrCl 3 2.3g (7.8mmol), 2-ethoxyethanol 60mL and H 2 O 20mL (2-ethoxyethanol / H 2 After adding O=3 / 1) into the flask, the mixture was refluxed and stirred at 120° C. for 24 hours. After completing the reaction, the mixture was washed with H 2 O / MeOH / Hex washing, and drying afforded compound 1-23.0 g (56%).
[0074] Preparation of Compound 1-3
[0075] Compound 1-23.0g (2.2mmol), 2,4-pentanedion (pentanedion) 0.6g (6.5mmol), Na 2 CO 3 After 1....
Embodiment 2
[0078] Embodiment 2: the preparation of compound D-2 and D-8
[0079]
[0080] Preparation of compound 2-1
[0081] 2,5-dibromopyridine (20g, 84mmol), 2,4-dimethylphenylboronic acid (15g, 101mmol), Pd(PPh 3 ) 4 4g (3.4mmol), Na 2 CO 3 (27g, 253mmol), toluene (240mL) and H 2 After O (120 mL) was added to the flask, the mixture was stirred at 100°C for 12 hours. The reaction mixture was then extracted with ethyl acetate (EA), washed with MgSO 4 Remove moisture and distill under reduced pressure. Then, the reaction mixture was dried and separated by column to obtain compound 2-118g (70%).
[0082] Preparation of Compound 2-2
[0083] Compound 2-2 (18 g, 99%) was prepared in a flask using compound 2-1 (18 g, 70 mmol) and phenylboronic acid (13 g, 105 mmol) in the same manner as the synthesis method of compound 1-1.
[0084] Preparation of compound 2-3
[0085] According to the same method as the synthetic method of compound 1-2, compound 2-2 (14%, 54 mmol) and...
Embodiment 3
[0090] Embodiment 3: the preparation of compound D-9 and D-10
[0091]
[0092] Preparation of compound 3-1
[0093] Compound 3-1 (16 g, 79%) was prepared in a flask using 2,5-dibromopyridine (20 g, 84 mmol) and phenylboronic acid (12 g, 101 mmol) in the same manner as that of compound 2-1.
[0094] Preparation of compound 3-2
[0095] Compound 3-2 (17g, 97%).
[0096] Preparation of compound 3-3
[0097] According to the same method as the synthetic method of compound 2-3, compound 3-2 (7g, 27mmol) and IrCl were used in a flask 3 (3.7 g, 12 mmol) Compound 3-3 (6 g, 65%) was prepared.
[0098] Preparation of Compound D-10
[0099] Compound D-10 (5g, 81% ).
[0100] Preparation of Compound D-9
[0101] Compound D-9 (1.6 g, 45%) was prepared in a flask using compound D-10 (3 g, 3.7 mmol) and compound 3-2 (2 g, 7.4 mmol) in the same manner as compound D-8. .
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com