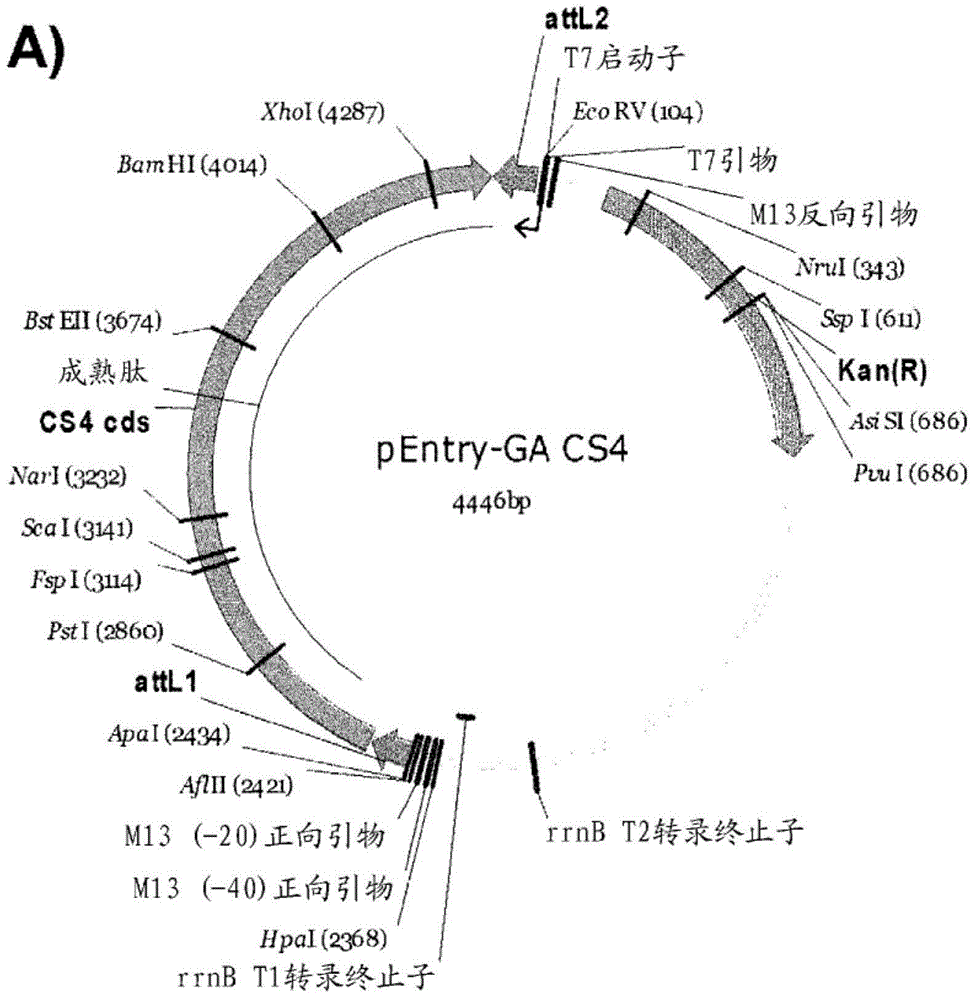
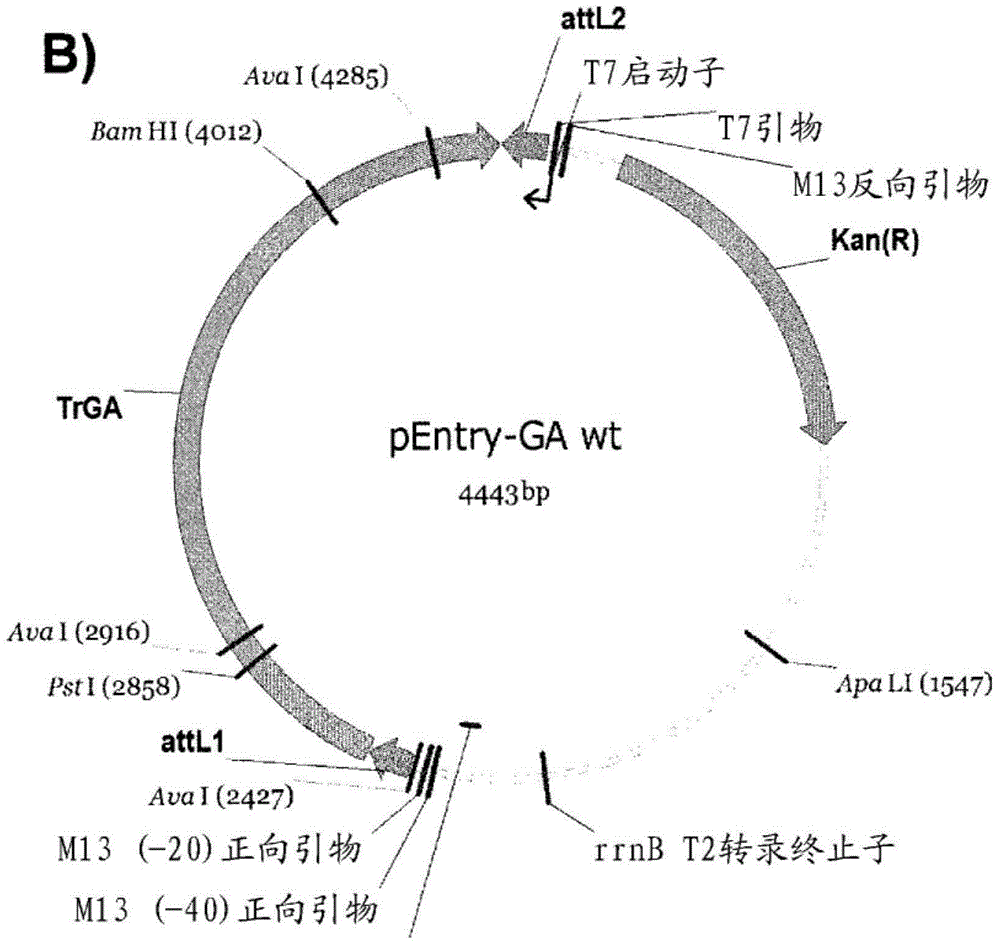
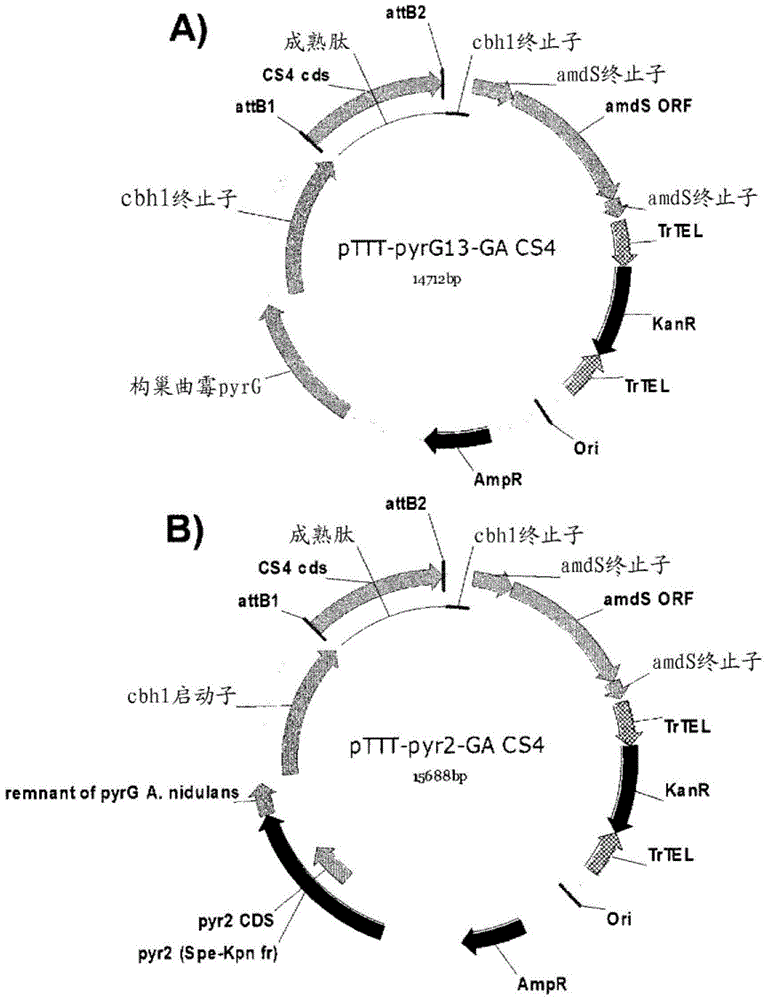
Variants having glucoamylase activity
A technology of glucoamylase and variant, applied in the direction of glycosylase, enzyme, hydrolase, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0075] Glucoamylases are commercially important enzymes in a wide range of applications requiring the hydrolysis of starch. Disclosed herein are glucoamylase variants with reduced thermostability for hydrolyzing starch. These glucoamylase variants contain amino acid substitutions in the catalytic domain and / or the starch binding domain. Said variants exhibit altered properties, such as altered thermostability and / or altered specific activity.
[0076] Furthermore, it is described herein that certain subsets of glucoamylase variants are very useful to add to fermentation vessels, for example, during beer fermentation, since the appropriate thermolabile nature of the enzyme allows inactivation by pasteurization become possible.
[0077] Pasteurization experiments have been performed on beer at laboratory scale, pilot scale and full scale to assess the ability to inactivate the variants described herein during brewing. Lab-scale pasteurization was demonstrated on bottled beer ...
Embodiment 1
[0451] Example 1: A glucoamylase variant comprising an interfacial amino acid group consisting of residues 29, 43, 48, 116 and 502 of SEQ ID NO: 2 or equivalent positions in the parent glucoamylase and in the set of catalytic core amino acid residues consisting of residues 97, 98, 147, 175, 483 and 484 of SEQ ID NO: 2 or equivalent positions in the parent glucoamylase One, two or three amino acid substitutions.
Embodiment 2
[0452] Embodiment 2: A glucoamylase variant comprising
[0453] a) Amino acid substitution at the residue corresponding to position 502 of SEQ ID NO: 2 or the equivalent position in the parent glucoamylase, and optionally, residues 29, 43 selected from SEQ ID NO: 2 , 48 and 116, or the amino acid substitution of the interface amino acid group consisting of equivalent positions in the parent glucoamylase;
[0454] b) amino acid substitutions at the residue corresponding to position 98 of SEQ ID NO: 2 or the equivalent position in the parent glucoamylase, and optionally, residues 97, 147 selected from SEQ ID NO: 2 , 175, 483, and 484, or one or two amino acid substitutions in the catalytic core amino acid residue set consisting of equivalent positions in the parent glucoamylase;
[0455] The glucoamylase variant has at least one amino acid substitution selected from the group of interface amino acid residues or the group of catalytic core amino acid residues;
[0456] wherein ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com