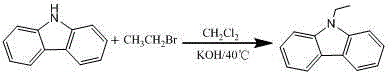Carbazolyl triarylamine hole transmission material and synthesis method thereof
A carbazolyl triarylamine, hole transport material technology, applied in the field of hole transport materials and their synthesis, can solve the problems affecting the application development of OLEDs, restrict the development of Mqn, etc., achieve excellent hole transport performance, and simple purification methods. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
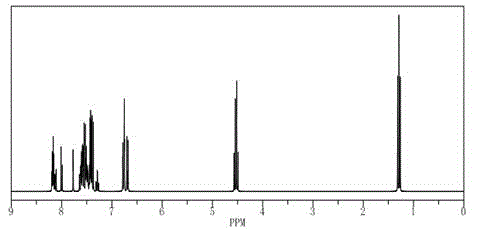
[0047] Below in conjunction with accompanying drawing two-[3-(9-ethyl)-9H-carbazolyl]-[4-(3-(9-phenyl)-9H-carbazolyl)-phenyl] provided by the present invention The preparation method of amine will be described.
[0048] Example of a synthetic route to bis-[3-(9-ethyl)-9H-carbazolyl]-[4-(3-(9-phenyl)-9H-carbazolyl)-phenyl]amine:
[0049] Synthetic two-[3-(9-ethyl)-9H-carbazolyl]-[4-(3-(9-phenyl)-9H-carbazolyl)-phenyl]amine according to following reaction formula [0013 ]
[0050]
[0051]
[0052]
[0053] Ethyl carbazole specific synthesis operation method: Weigh 166.2 g carbazole, 112 g KOH, and then measure 831 ml dichloromethane, add it to a 3 L reaction three-necked flask, slowly raise the temperature to 40 °C under mechanical stirring, and wait until the carbazole is completely dissolved , Start to add 114.3g of bromoethane dropwise, keep the temperature at 30°C-40°C, and the dropwise addition time is 2~4h. When the carbazole content is less than 0.5%, the rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com