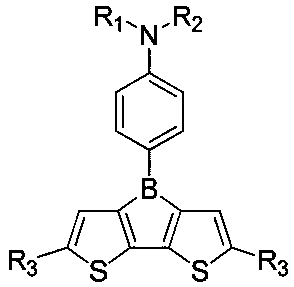
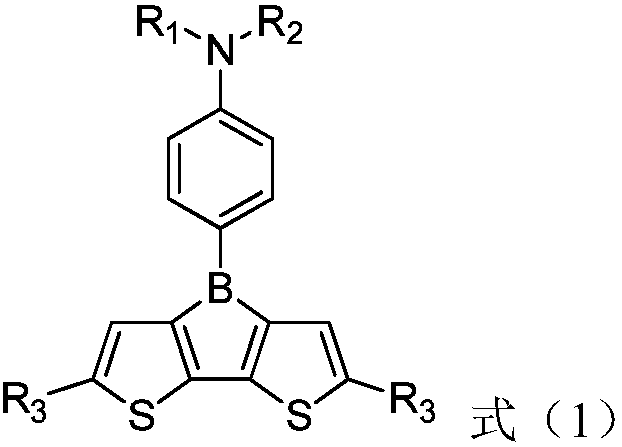
Organic photoelectric material with boron bridged 2,2'-dithiophene structure and application of organic photoelectric material
An organic optoelectronic material, dithiophene technology, which is applied in the field of organic optoelectronic materials with boron-bridged dithiophene structure, can solve the problems of insufficient development of OLED materials, lagging behind the requirements of panel manufacturing enterprises, and shortage, etc., so as to facilitate the transmission of electrons , Improve electron affinity, improve the effect of molecular stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
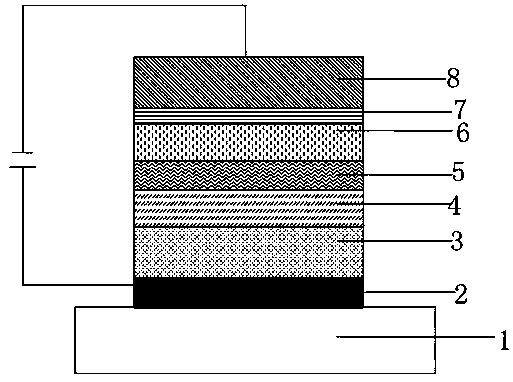
Image
Examples
Embodiment 1
[0049] Embodiment 1. Preparation of organic optoelectronic materials
Embodiment 11
[0050] The synthesis of embodiment 11 compound 1:
[0051] Dissolve 3,3'-dibromo-5,5'-di-tert-butyl-2,2'-bithiophene (4.36g, 10.0mmol) in 100mLTHF, cool down to -70°C, add n-butyllithium dropwise n-Hexane solution (12mL, 2.0mol / L), after the dripping, keep warm for 1.0hrs. Control the internal temperature to less than -70°C, add a THF solution of 4-(boron dichloride)-N,N-diphenylamine aniline (3.5g, 11mmol, 0.1mol / L) dropwise, and keep it warm for 3.0hrs after the dropping , slowly warming up to room temperature, poured into 100g saturated aqueous sodium chloride solution, layered, the organic phase was washed with saturated aqueous sodium chloride solution, dried, and desolvated to obtain 5.6g of a brown-yellow viscous liquid. Recrystallized using n-hexane to obtain off-white solid particles (2.9 g, yield 54.61%). Using DEI-MS to identify the compound, formula C 34 h 34 BNS 2 , detection value [M+1] + =532.49, calculated value 531.58.
Embodiment 12
[0052] The synthesis of embodiment 12 compound 2:
[0053] Dissolve 3,3'-dibromo-5,5'-di-tert-butyl-2,2'-bithiophene (4.36g, 10.0mmol) in 100mLTHF, cool down to -70°C, add n-butyllithium dropwise n-Hexane solution (12mL, 2.0mol / L), after the dripping, keep warm for 1.0hrs. Control the internal temperature to less than -70°C, add 4-(boron dichloride)-N,N-bis(4-isopropylphenyl)aniline THF solution (4.5g, 11mmol, 0.1mol / L) dropwise, drop After completion, keep the reaction for 3.0 hrs, slowly warm up to room temperature, pour into 100 g of saturated aqueous sodium chloride solution, separate layers, wash the organic phase with saturated aqueous sodium chloride solution, dry, and remove the solvent to obtain 6.5 g of brown-yellow viscous liquid. Recrystallized using n-hexane to obtain off-white solid particles (3.4 g, yield 55.28%). Using DEI-MS to identify the compound, formula C 40 h 46 BNS 2 , detection value [M+1] + =615.79, calculated value 615.74.
[0054] Compounds 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com