Active peptide capable of being specifically combined with TfR1 and application of active peptide
An active peptide, specific technology, applied in the field of active peptides, can solve problems such as limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Through the method of phage display, the transferrin receptor-specific binding peptide CPU-312-01 was obtained:
[0033](1) The nucleotide sequence of the transferrin receptor-specific binding peptide CPU-312-01 was obtained by screening the phage random peptide library:
[0034] A. Biopanning of phage random 7-peptide library: Dilute the transferrin receptor to 100 μg / ml with coating solution (0.1 M NaHCO3, pH 8.6), add 150ul to one well of the microplate In the middle, rotate repeatedly until the surface is completely wet, keep wet, 4 ℃ overnight. Pour off the coating solution, clap the plate to remove the residual solution, fill up with blocking solution (0.1 M NaHCO3, pH 8.6, 5 mg / ml BSA), and react at 4°C for at least one hour. Pour out the blocking solution, wash 6 times with 0.1% TBST [TBS + 0.1% (v / v) Tween-20], and tap the plate each time. Dilute 10 μl of the original phage peptide library with 100 μl TBST, add it to the well coated with transferrin receptor,...
Embodiment 2
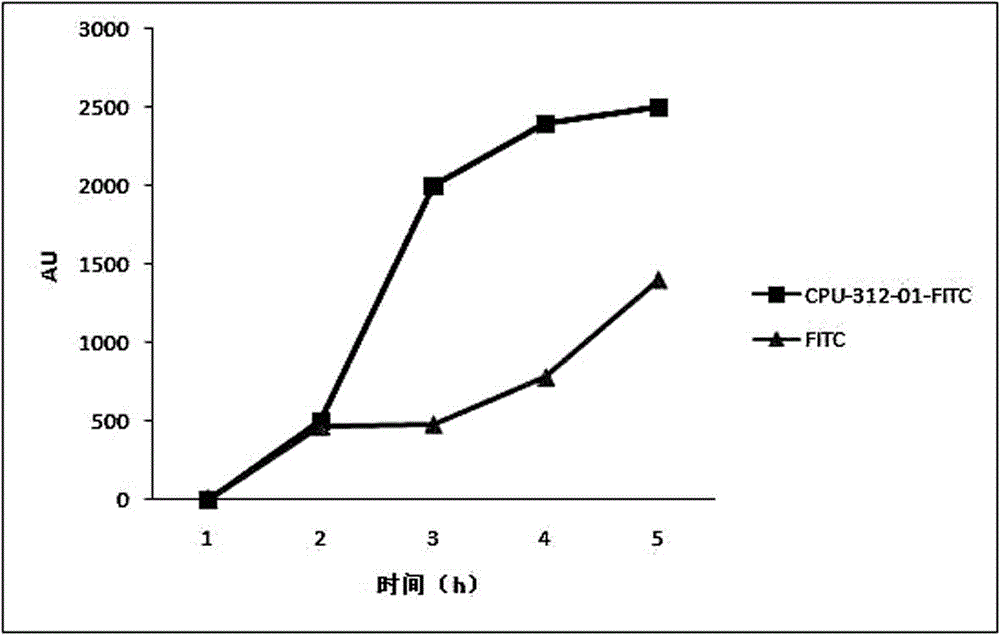
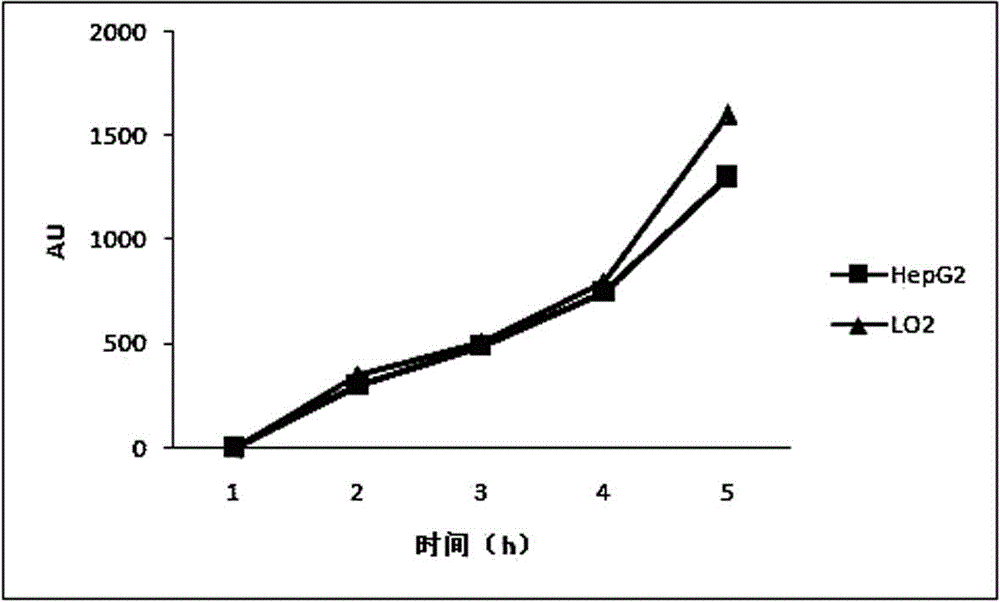
[0043] Example 2 Fluorescent microplate reader detects the uptake experiment of cells coupled with FITC-binding peptide and FITC:
[0044] Take HepG2 cells (liver cancer cells) in the logarithmic growth phase, digest with an appropriate amount of 0.25% trypsin solution, and shake gently to make the digestive juice evenly act on the cells. RPMI-1640 medium with 10% calf serum, pipet gently with a pipette to form a single cell suspension; add 8×10 4 cells in a 24-well plate, and add 500 μl RPMI-1640 medium containing 10% serum, at 37 °C, containing 5 % CO 2 Cultivate in a constant temperature incubator for 24 hours to the state of monolayer cells; 1h, 2h, 3h, 4h before the test, add 500μl of RPMI-1640 medium containing 10% calf serum, 50um coupled with FITC-binding peptide, each time Spot 3 replicate wells and place at 37°C, 5% C0 2 Cultured in a constant temperature incubator; at the same time, FITC and LO2 cells (normal liver cells) were used as negative controls, and th...
Embodiment 4
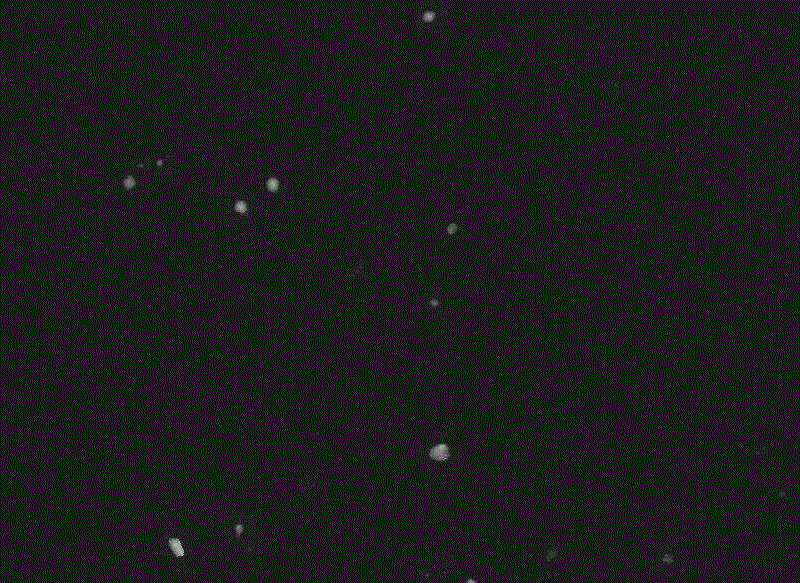
[0045] Example 4 Fluorescent microscope observation of phagocytosis of cells coupled to FITC-binding peptides and FITC:
[0046] Take HepG2 cells (liver cancer cells) in the logarithmic growth phase, digest with an appropriate amount of 0.25% trypsin solution, and shake gently to make the digestive juice evenly act on the cells. RPMI-1640 medium with 10% calf serum, pipette gently to make a single cell suspension, add 8×10 4 cells in a 24-well plate, and add 500 μl RPMI-1640 medium containing 10% serum, at 37 °C, containing 5 % CO 2 Cultivate in a constant temperature incubator for 24 hours to the state of monolayer cells, add 500 μl of RPMI-1640 medium containing 10% calf serum, 50um coupled FITC-binding peptide to each well, and place in 3 duplicate wells at 37°C, 5% C0 2 Cultured in a constant temperature incubator; at the same time, FITC and LO2 cells (normal liver cells) were used as negative controls. After 4 hours, the medium was aspirated, washed twice with PBS,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com