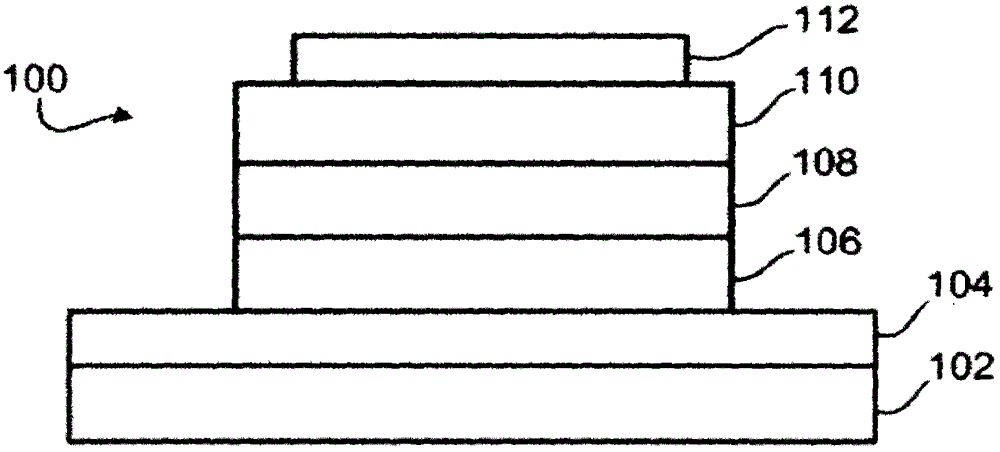
Platinum complexes and devices
A compound and independent technology, applied in the field of platinum complexes, can solve the problems of poor processability, less than ideal stability, low efficiency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
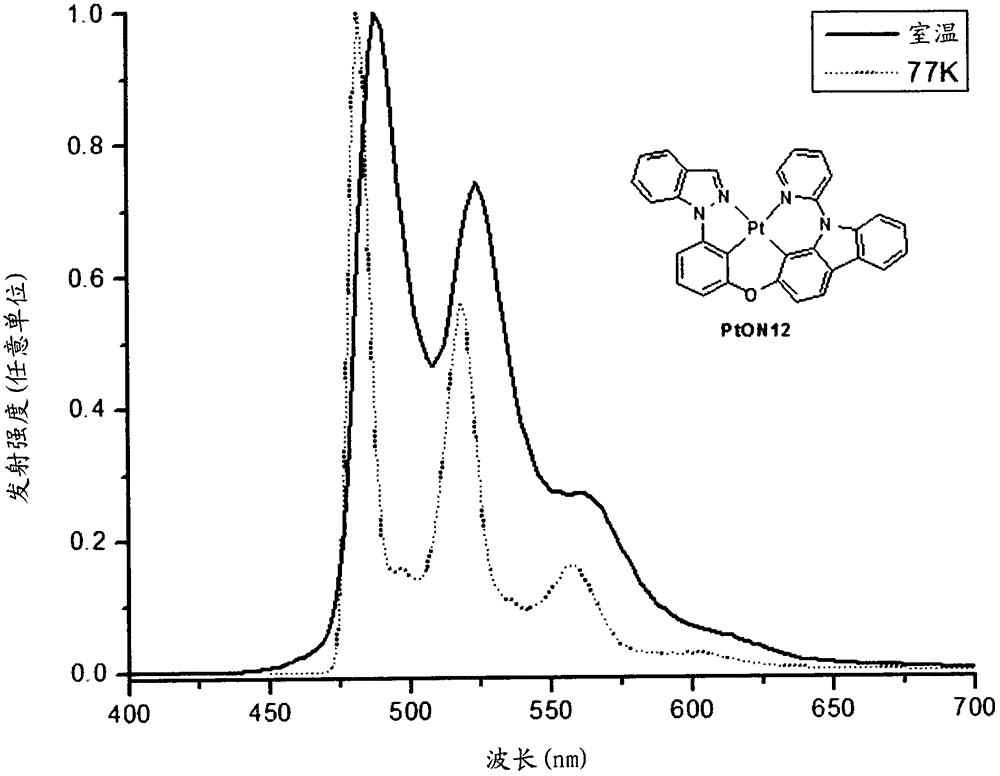
[0446] The platinum complex PtON12 was prepared according to the following scheme:
[0447]
[0448] Synthesis of 1-(3-methoxyphenyl)-1H-indazole 1:
[0449]
[0450] To a dry pressure tube equipped with a magnetic stir bar was added 1H-indazole (3.54 g, 30 mmol, 1.0 equiv), 1-iodo-3-methoxybenzene (8.07 g, 36 mmol, 1.2 equiv), CuI (0.29 g , 1.5mmol, 0.05 equivalent), K 2 CO 3 (13.37 g, 63 mmol, 2.1 equiv) and trans-1,2-cyclohexanediamine (0.65 g, 6 mmol, 0.2 equiv). The tube was then taken into the glove box, and the solvent toluene (40 mL) was added. The mixture was bubbled with nitrogen for 5 minutes and the tube was sealed. The tube was removed from the glove box, and the mixture was stirred in an oil bath at 105-115°C for 3 days. The mixture was cooled to ambient temperature, diluted with ethyl acetate, then filtered and washed with ethyl acetate. The filtrate was concentrated and the residue was purified by column chromatography on silica gel using hexane and...
Embodiment 2
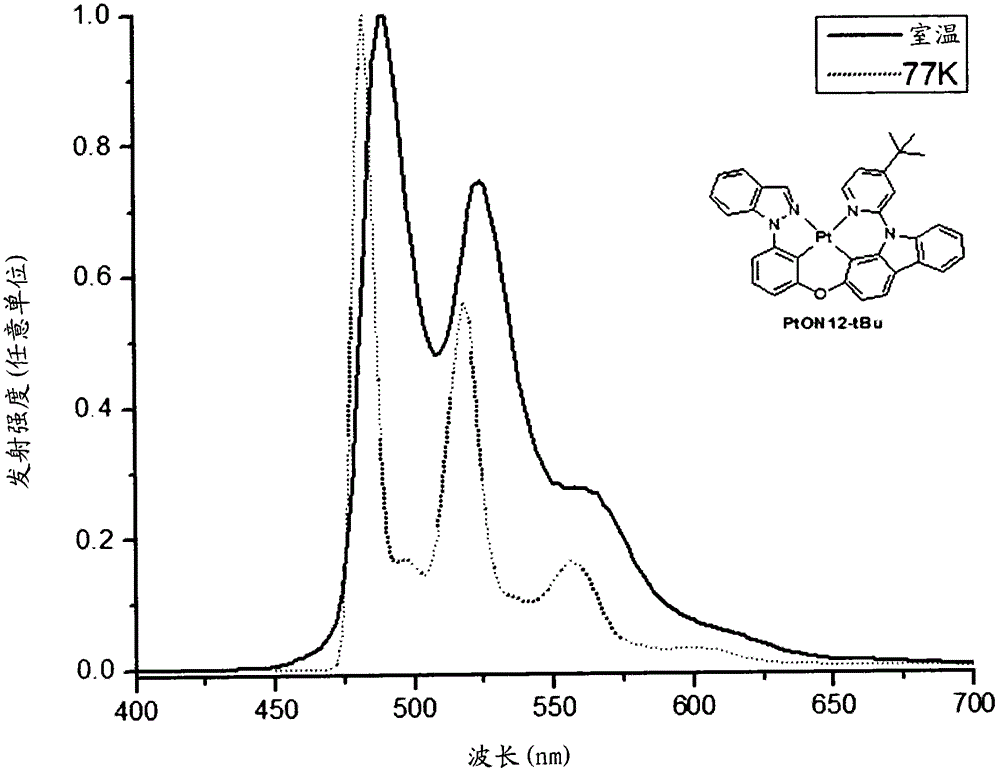
[0462] The platinum complex PtON12-tBu was prepared according to the following scheme:
[0463]
[0464] Synthesis of 2-(3-(1H-indazol-1-yl)phenoxy)-9-(4-tert-butylpyridin-2-yl)-9H-carbazole ligand ON12-tBu:
[0465]
[0466] To a dry Shlenck tube fitted with a magnetic stir bar was added 3-(1H-indazol-1-yl)phenol 2 (630 mg, 3.0 mmol, 1.0 equiv), 9-(4-tert-butylpyridin-2-yl) -2-Bromo-9H-carbazole (1365mg, 3.6mmol, 1.2eq), CuI (57mg, 0.3mmol, 0.1eq), picolinic acid (74mg, 0.6mmol, 0.2eq) and K 3 PO 4 (1273 mg, 6.0 mmol, 2.0 equiv). The tube was evacuated and backfilled with nitrogen. The vacuum and backfill procedure was repeated two more times. Then the solvent DMSO (9 mL) was added under nitrogen protection. The mixture was stirred in an oil bath at a temperature of 90-100° C. for 3 days and then cooled to ambient temperature. Water was added to dissolve the solids. The mixture was extracted three times with ethyl acetate. The combined organic layers were washed...
Embodiment 3
[0472] The platinum complex PtON13 can be prepared according to the following scheme:
[0473]
[0474] Synthesis of 4-bromo-1-(3-methoxyphenyl)-1H-pyrazole 3:
[0475]
[0476] To a dry pressure tube equipped with a magnetic stir bar was added 4-bromo-1H-pyrazole (3674 mg, 25 mmol, 1.0 eq), CuI (95 mg, 0.5 mmol, 0.02 eq) and K 2 CO 3 (7256 mg, 52.5 mmol, 2.1 equiv). Then trans-1,2-cyclohexanediamine (570 mg, 5 mmol, 0.2 equiv), 1-iodo-3-methoxybenzene (3.57 mL, 30 mmol, 1.2 equiv) and Solvent dioxane (50 mL). The mixture was bubbled with nitrogen for 5 minutes. Seal the tube before removing it from the glove box. The mixture was stirred at a temperature of 100° C. in an oil bath for two days. The mixture was then cooled to ambient temperature, filtered, and washed with ethyl acetate. The filtrate was concentrated and the residue was purified by column chromatography on silica gel using hexane and ethyl acetate (20:1-15:1) as eluent to give the desired product 4-b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com