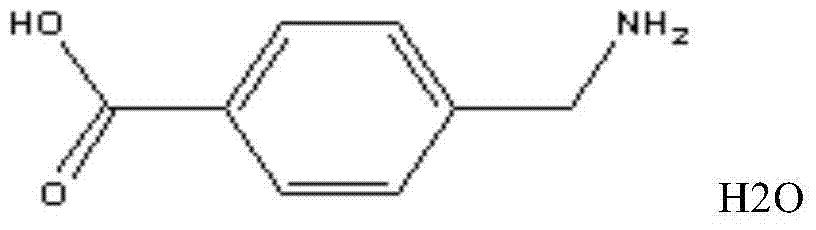
Aminomethylbenzoic acid freeze-dried powder injection medicine composition for injection
A technology of freeze-dried powder injection and aminotoluic acid, which is applied in the field of aminotoluic acid freeze-dried powder injection pharmaceutical composition for injection, and can solve problems such as inability to block
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0082] Preparation Example 1, Preparation of Powder Injection Containing Aminotoluic Acid
[0083] formula:
[0084] Aminomethylbenzoic acid
100mg,
95 mg,
Dextran-20
5 mg,
pH regulator
to pH4.0,
Water for Injection
Appropriate amount, add to 4ml.
[0085] Preparation:
[0086] (1) Take the principal agent and auxiliary materials of the prescription quantity, place in a stainless steel bucket, add about 80% of the prescription quantity of water for injection, make each component dissolve, then add 0.1% (w / v) gac by the volume of the solution, Stir for 30 minutes, filter and decarburize, and add water for injection to nearly the full amount of the prescription.
[0087] (2) The filtrate is sampled, and the pH value is measured, and if necessary, it is adjusted to a specified value with a pH regulator (this specified value is the value of the measured pH value of the freeze-dried gained dry powder dilute...
preparation example 2
[0091] Preparation Example 2, Preparation of Powder Injection Containing Aminotoluic Acid
[0092] formula:
[0093] Aminomethylbenzoic acid
100mg,
50mg,
Dextran-20
10mg,
pH regulator
to pH4.5,
Water for Injection
Appropriate amount, add to 6ml.
[0094] Preparation:
[0095] (1) Take the principal agent and auxiliary materials of the prescription quantity, place in a stainless steel bucket, add about 80% of the prescription quantity of water for injection, make each component dissolve, then add 0.1% (w / v) gac by the volume of the solution, Stir for 30 minutes, filter and decarburize, and add water for injection to nearly the full amount of the prescription.
[0096] (2) The filtrate is sampled, and the pH value is measured, and if necessary, it is adjusted to a specified value with a pH regulator (this specified value is the value of the measured pH value of the freeze-dried gained dry powder dilute...
preparation example 3
[0100] Preparation Example 3, Preparation of Powder Injection Containing Aminotoluic Acid
[0101] formula:
[0102] Aminomethylbenzoic acid
100mg,
150mg,
Dextran-40
2 mg,
pH regulator
to pH3.5,
Water for Injection
Appropriate amount, add to 5ml.
[0103] Preparation:
[0104] (1) Take the principal agent and auxiliary materials of the prescription quantity, place in a stainless steel bucket, add about 80% of the prescription quantity of water for injection, make each component dissolve, then add 0.1% (w / v) gac by the volume of the solution, Stir for 30 minutes, filter and decarburize, and add water for injection to nearly the full amount of the prescription.
[0105] (2) The filtrate is sampled, and the pH value is measured, and if necessary, it is adjusted to a specified value with a pH regulator (this specified value is the value of the measured pH value of the freeze-dried gained dry powder diluted...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com