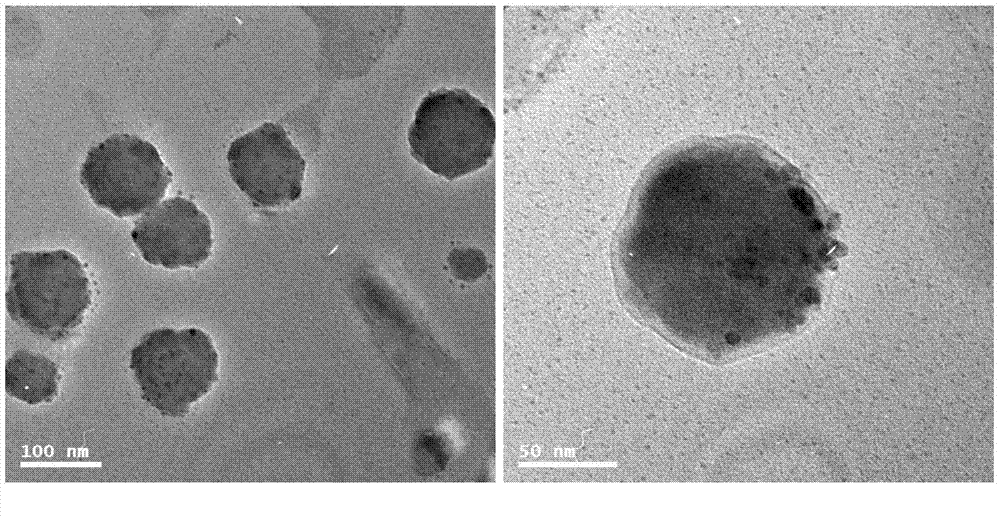
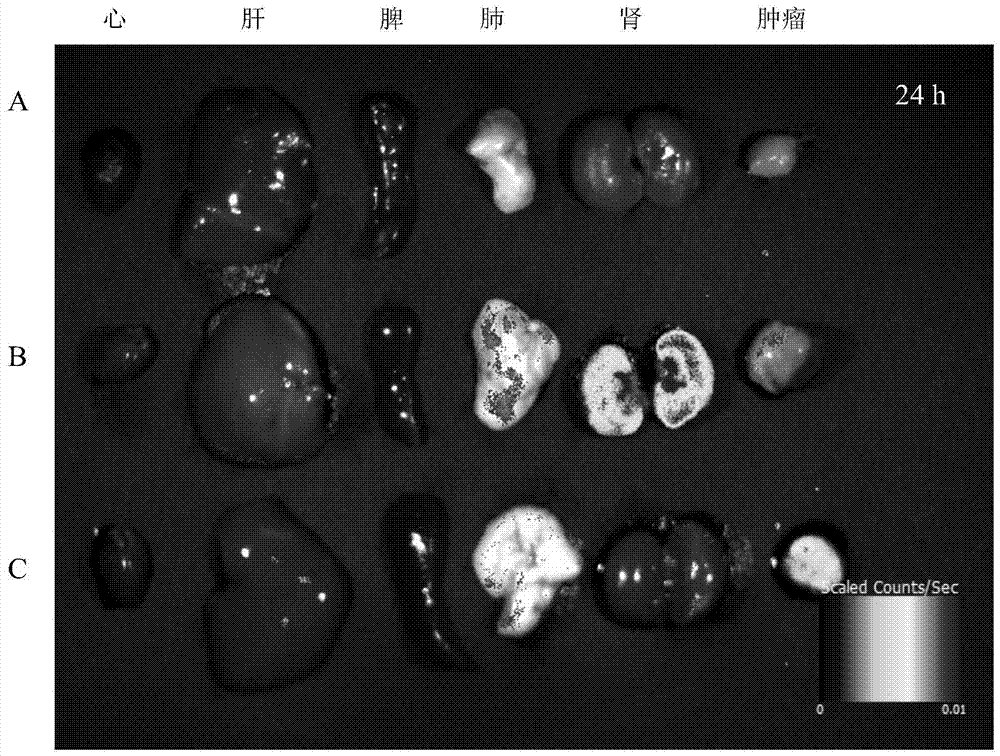
Polymersome with hydrophilic lumen carrying anthracene ring medicines as well as preparation method and application
An anthracycline and polymer technology is applied in the field of drug carriers to achieve the effects of good in-vitro stability and high drug encapsulation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A kind of polycaprolactone-b-polyethylene glycol-b-polycaprolactone (PCL-b-PEG-b-PCL) amphiphilic triblock copolymer is made by following method: (PCL 2000 -b-PEG 2000 -b-PCL 2000 )
[0036] (1) ε-caprolactone (ε-CL) by calcium hydride (CaH 2 ) is dried and distilled under reduced pressure for subsequent use; polyethylene glycol 2000 (PEG, molecular weight 2000) is azeotropically dehydrated with toluene;
[0037] (2) Weigh 5g PEG 2000 and 10g ε-CL and add them to the dry polymerization tube treated by silanization, add stannous octoate as catalyst, and the added mass of the catalyst is the reactant (PEG 2000 and ε-CL) 0.1% of the mass, and then the system was vacuum-filled with nitrogen for 3 times, vacuum-sealed, and placed in a 120°C constant temperature oil bath to react for 12 hours to obtain a polymer;
[0038] (3) Dissolve the polymer in a small amount of dichloromethane, pour a large amount of petroleum ether for re-precipitation, and vacuum-dry the precipita...
Embodiment 2
[0040] a PCL 4000 -b-PEG 4000 -b-PCL 4000 Amphiphilic triblock copolymers prepared by the following method:
[0041] (1) ε-CL via CaH 2 Dry and distill under reduced pressure for later use; PEG 4000 is azeotropically dehydrated with toluene;
[0042] (2) Weigh 5g of PEG 4000 and 10g of ε-CL and add them to the dry polymerization tube treated with silanization, add stannous octoate as catalyst, and the added mass of the catalyst is the reactant (PEG 4000 and ε-CL) 0.1% of the mass, and then the system was vacuum-filled with nitrogen for 3 times, vacuum-sealed, and placed in a 160°C constant temperature oil bath to react for 16 hours to obtain a polymer;
[0043] (3) Dissolve the polymer in a small amount of dichloromethane, pour a large amount of anhydrous ether for reprecipitation, and vacuum dry the precipitate at 40°C to constant weight to obtain PCL 4000 -b-PEG 4000 -b-PCL 4000 Amphiphilic triblock copolymers.
Embodiment 3
[0045] a PCL 6000 -b-PEG 6000 -b-PCL 6000 Amphiphilic triblock copolymers prepared by the following method:
[0046] (1) ε-CL via CaH 2 Dry and distill under reduced pressure for later use; PEG 6000 is azeotropically dehydrated with toluene;
[0047] (2) Weigh 5g PEG 6000 and 10g ε-CL and add them to the dry polymerization tube treated by silanization, add stannous octoate as catalyst, and the addition quality of the catalyst is the reactant (PEG 6000 and ε-CL) 0.1% of the mass, and then the system was vacuum-filled with nitrogen for 3 times, vacuum-sealed, and placed in a 160°C constant temperature oil bath to react for 16 hours to obtain a polymer;
[0048] (3) Dissolve the polymer in a small amount of dichloromethane, pour a large amount of anhydrous methanol for reprecipitation, and vacuum dry the precipitate at 40°C to constant weight to obtain PCL 6000 -b-PEG 6000 -b-PCL 6000 Amphiphilic triblock copolymers.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com