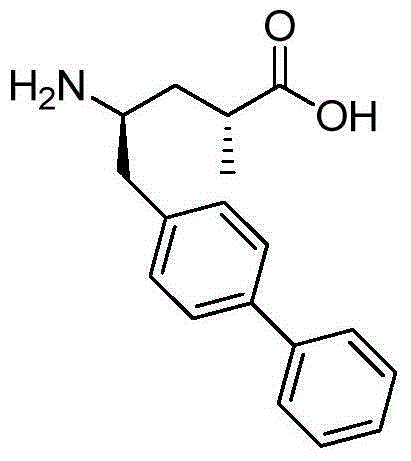
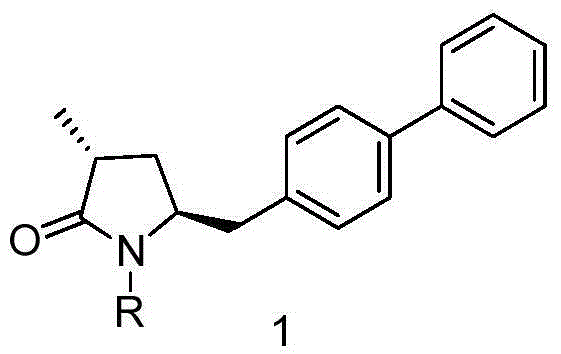
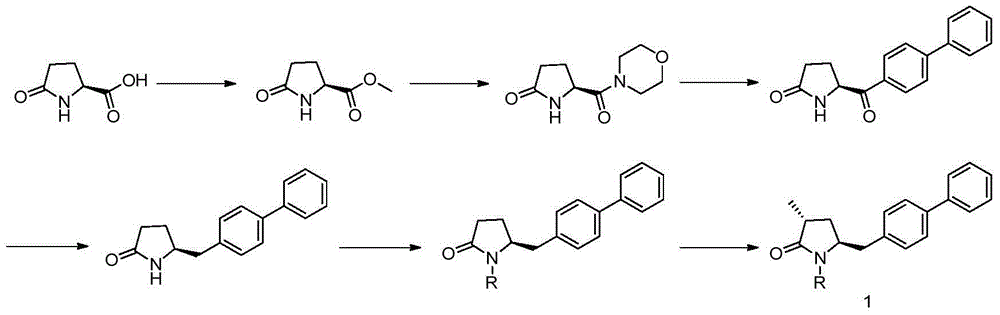
Synthesis method of 5-biphenyl-4-amino-2-methylvaleric acid intermediate
A synthesis method and methyl technology, applied in the field of medicine, can solve the problems of low yield, poor methylation selectivity, difficult separation of diastereomers, etc., so as to solve the problem of poor reaction selectivity and good selectivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Synthesis of compound (5a)
[0070]
[0071] Add 288g of compound 6 into 1.5L of dichloromethane, add 476g of p-toluenesulfonyl chloride and 25g of DMAP, drop the temperature to 0°C, add 610g of triethylamine dropwise, and stir at room temperature for 3 hours after dropping. The reaction solution was washed with water, washed with acid, dried, concentrated, and slurried by adding methyl tert-butyl ether to obtain 545 g of compound 5a.
Embodiment 2
[0073] Synthesis of compound (4a)
[0074]
[0075] Add 190g of 5a into 1L of ethyl acetate, add 169g of Boc-anhydride, 8.7g of DMAP, and heat to 50°C for 5 hours. The reaction solution was washed with water, dried and concentrated to obtain 243 g of compound 4a, with a yield of 96.4%.
Embodiment 3
[0077] Synthesis of compound (4b)
[0078]
[0079] 190g of 5a was added to 1L of ethyl acetate, 8.7g of DMAP and 86g of triethylamine were added, 94g of pivaloyl chloride was added dropwise at 0°C, and the reaction was completed for 3 hours. The reaction solution was washed with water, washed with acid, dried and concentrated to obtain 235 g of compound 4a with a yield of 93.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com