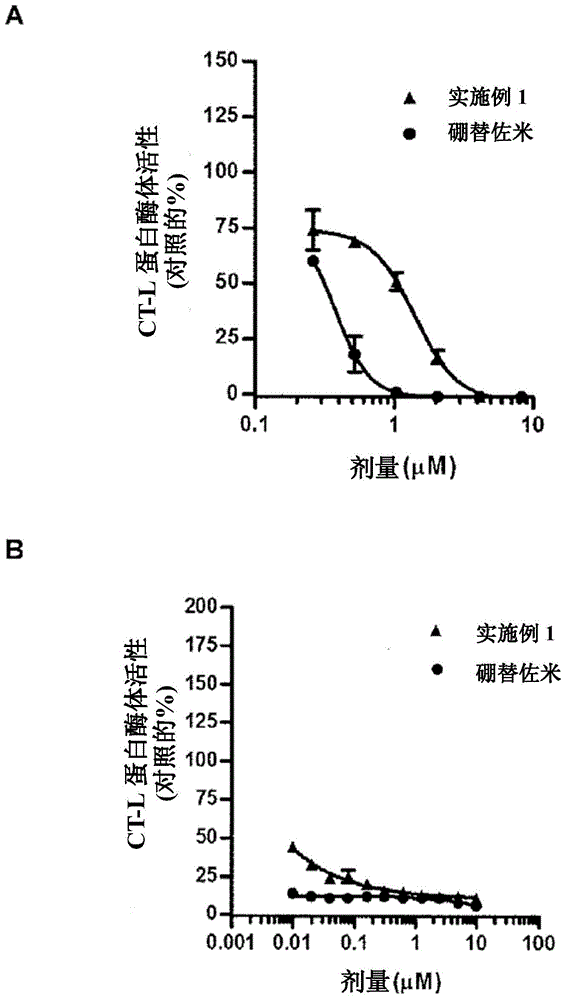
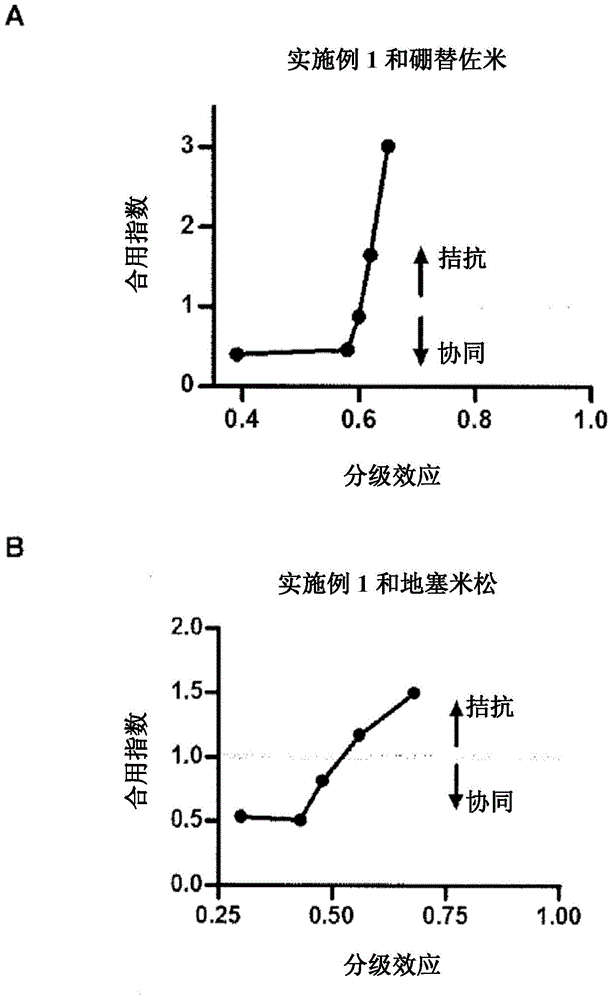
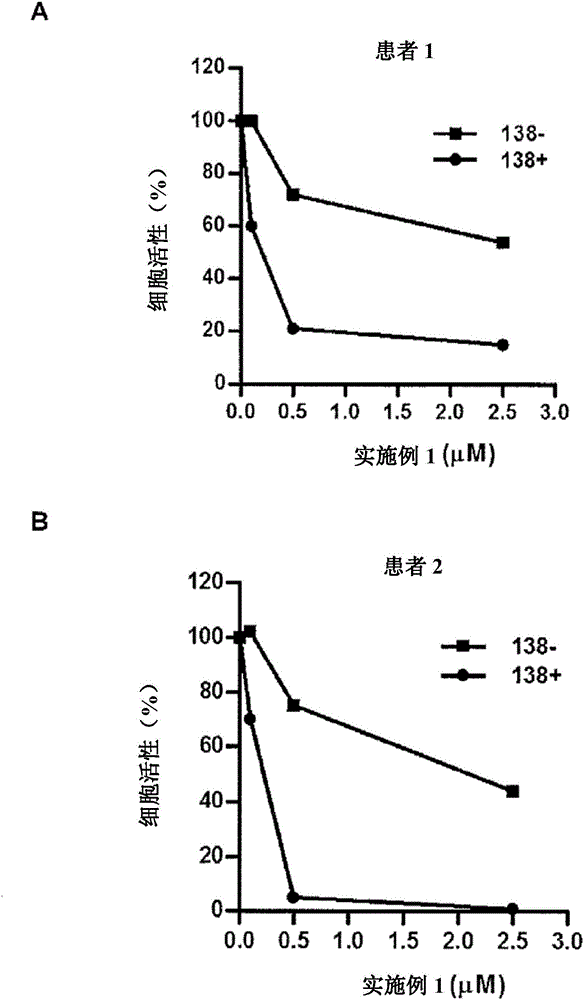
Fluorinated epoxyketone-based compounds and uses thereof as proteasome inhibitors
A compound and solvate technology, applied in the field of novel fluorinated epoxy ketone series compounds, can solve problems such as damage or burden cell cycle control and abnormal regulation of signal transduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0318] III. Preparation of the compounds of the present application
[0319]The compounds of the present application can be prepared by a variety of synthetic methods. The choice of particular structural features and / or substituents can influence the choice of one approach over another. The choice of a particular method for preparing a given compound of formula I is within the skill of the art. Some of the starting materials used to prepare the compounds of the present application can be obtained from commercial chemical sources. Other starting materials, such as those described below, are readily prepared from available precursors using concise transformations well known in the art.
[0320] In one embodiment, compounds of formula I are generally prepared according to the method shown in Scheme I. The variables in the following schemes are as defined above for formula I unless otherwise specified.
[0321]
[0322] route I
[0323] In one embodiment, as shown in Sch...
Embodiment 1
[0480] Scheme XIII outlines the compound of formula I of Example 1 2-methylthiazole-5-carboxylic acid ((S)-1-{(S)-1-[(S)-1-benzyl-2-(( R)-2-Methyloxinyl)-2-oxoethylcarbamoyl]-2-difluoromethoxyethylcarbamoyl}-2-difluoromethoxyethyl)amide preparation.
[0481]
[0482] Route XIII
[0483] Reagents and conditions for route XIII: (i) HBTU, HOBt, DIPEA, THF, 0°C to RT / ON; (ii) H 2 , Pd / C (10%), THF, RT / 2hrs.; (iii) HBTU, HOBt, DIPEA, THF, 2-methylthiazole-5-carboxylic acid, 0°C to RT / ON.
[0484] (a)((S)-1-{(S)-1-[(S)-1-benzyl-2-((R)-2-methyloxopropyl)-2-oxoethyl Preparation of tert-butylcarbamoyl]-2-difluoromethoxyethylcarbamoyl}-2-difluoromethoxyethyl)carbamate (XIV(a)):
[0485]
[0486] To compound of formula II(b)(i) at 0°C (S)-2-((S)-2-tert-butoxycarbonylamino-3-difluoromethoxypropionylamino)-3-methoxy propionic acid (2.75 g, 7.028 mmol) and compound of formula S,R-III(a) (S)-2-amino-1-((R)-2-methyloxopropyl)-3- Phenylpropan-1-one trifluoroacetate (12.04 g, 6.389 ...
Embodiment 25
[0497] The compound of formula I of Example 25 (2S)-2-[[(2S)-3-(difluoromethoxy)-2-[(2-morpholinoacetyl)amino] was prepared according to the following synthetic procedure Propionyl]amino]-N-[(1S)-3-methyl-1-[(2R)-2-methyloxirane-2-carbonyl]butyl]-3-phenylpropanamide.
[0498] (a) Preparation of tert-butyl 2-morpholinoacetate:
[0499]
[0500] To a stirred solution of tert-butyl bromoacetate (8.47 mL, 57.4 mmol) in THF (50 mL) was added dropwise a 1:1 mixture of triethylamine (8 mL, 57.4 mmol) and morpholine (5.02 mL, 57.4 mmol) (observed to a slight exotherm) and the resulting white suspension was stirred at 60 °C for 2 h. The mixture was diluted with water (100 mL) and saturated sodium carbonate (50 mL) and extracted with ethyl acetate (2 x 50 mL). The combined organics were washed with saturated sodium carbonate (100 mL), water (3 x 50 mL) and brine (50 mL). The organic phase was dried over anhydrous sodium sulfate, filtered and concentrated, then chromatographed in h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com