Recombinant erythropoietin hepatocyte receptor a2 antagonist and its coding gene and application
An antagonist, hepatocyte technology, applied in cytokines/lymphokines/interferons, applications, genetic engineering, etc., can solve the problems of unsatisfactory results and achieve the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1 The design of ephrinA1 mimetic peptide
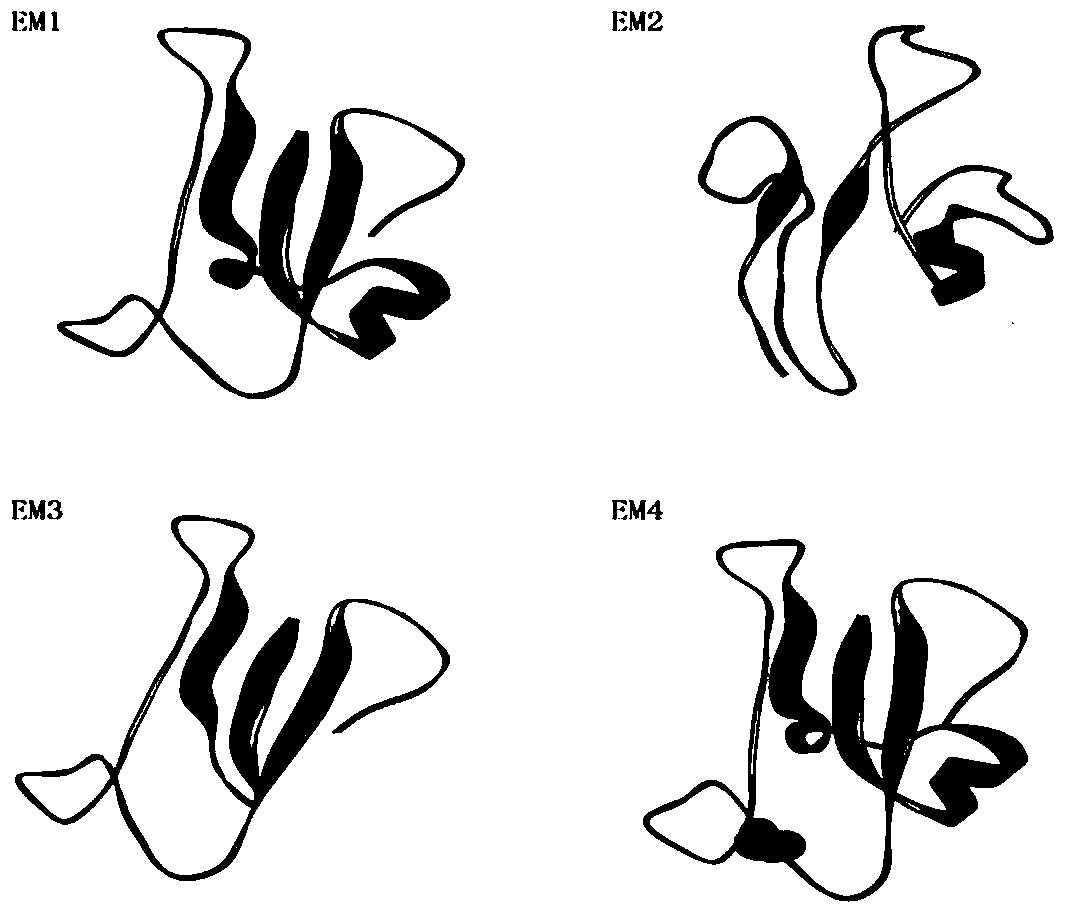
[0048] The purpose of the polypeptide design of the present invention is to find an antagonist of EphA2, and the mode of action of the antagonist is to compete with the natural ligand ephrinA1 of EphA2 for the binding site on the EphA2 receptor ( figure 1 ), thereby blocking the interaction between ephrinA1 and EphA2 receptors, thereby inhibiting the activation of EphA2. Therefore, the present invention uses ephrinA1 as the backbone to mutate, and retains the amino acid sequence for binding or replaces the amino acid sequence for activation to find a polypeptide that can interact with the EphA2 ligand binding site but has no activation effect itself.
[0049]1. The inventor of the present invention obtained the target gene (PDB ID: 3HEI) of the human ephrinA1 protein from the NCBI protein library website (http: / / www.ncbi.nlm.nih.gov / protein / P20827.2), and intercepted Amino acids related to binding were discarded, an...
Embodiment 2
[0054] Example 2 Synthesis of cDNA molecules encoding EM1-EM4 proteins
[0055] 1. Design the gene sequences encoding EM1-EM4 according to the preferred codons of Escherichia coli, as follows:
[0056] EM1
[0057] CATATGTATATTCTGTATCTGGTGGAACATGAAGAATATCAGCTGTGCCAGCCGCAGAGCAAAGATCAGGTGCGTTGGCAGTGCAATCGTCCGAGCGAAACATGGCCCGGAGAAACTGAGCGAGAAATTTCAGCGCTTTACGCCGTTTACCCTGGGCAAAGAATTTAAAGAAGGCCATAGCTATTATTATTAGCAAACCGGGCGGCAGCCTCGAG
[0058] EM2
[0059] CATATGTATATTCTGTATCTGGTGGAACATGAAGAATATCAGCTGTGCCAGCCGCAGAGCAAAGATCAGGTGCGTTGGCAGTGCAATCGTCCGAGCGCGAAACATGGCCCGGAGAAACTGAGCGAGAAATTTCAGAGCTGGCTGGCGTATCCGGGCGCGGTGAGCTATCGTGAAGGCCATAGCTATTATTATTAGCAAACCGGGCGGCAGCCTCGAG
[0060] EM3
[0061] CATATGTATATTCTGTATCTGGTGGGCGGCGGCAGCAGCGTGCGTTGGCAGTGCAATCGTCCGAGCGCGAAACATGGCCCGGAGAAACTGAGCGAGAAATTTCAGCGCTTTACGCCGTTTACCCTGGGCAAAGAATTAAAAGAAGGCCATAGCTATTATTATTAGCAAACCGGGCGGCAGCCTCGAG
[0062] EM4
[0063] CATATGTATATTCTGTATCTGGTGGAACATGAAGAATATCAGCTGTGCCAGCCGCAGAGCAAAGATCAGGTGCGTTGGCAGTG...
Embodiment 3
[0080] The construction of embodiment 3 expression vector
[0081] The experimental method in this experiment is mainly operated according to the product instructions provided by J. Sambrook and the company in "Molecular Cloning Experiment Guide" (third edition).
[0082] 1. The pET30a vector (purchased from Invitrogen) and the target fragment (cDNA synthesized in Example 2) were double digested with NdeI and XhoI;
[0083] 2. T4 ligase connection;
[0084] 3. Transform competent Escherichia coli E.coli tranT1, and screen positive clones with kanamycin medium;
[0085] 4. Cultivate positive bacteria;
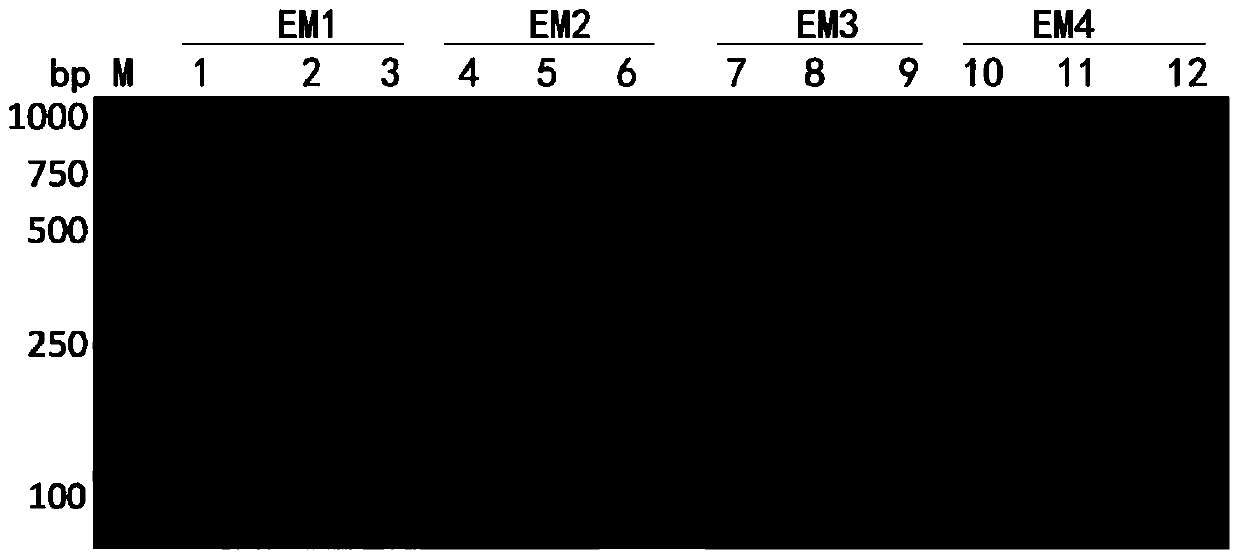
[0086] 5. Extract and purify the plasmid, and the electrophoresis results of the recombinant plasmid after double enzyme digestion are as follows: Figure 4 As shown in , the shorter one in the figure is the target fragment, and the longer one is the plasmid fragment, indicating that the recombinant plasmid was successfully constructed;
[0087] 6. Further sequencing was car...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com