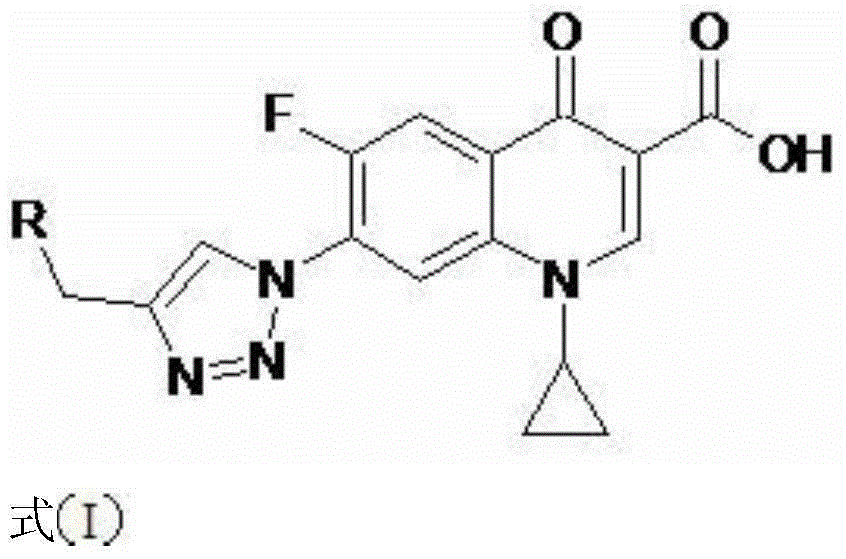
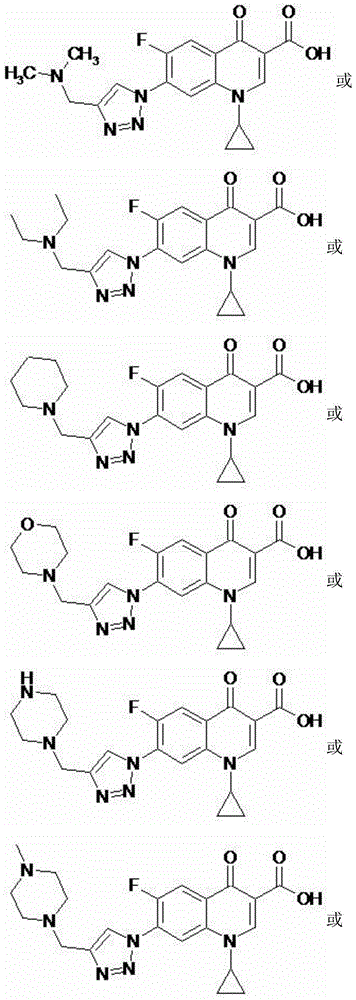
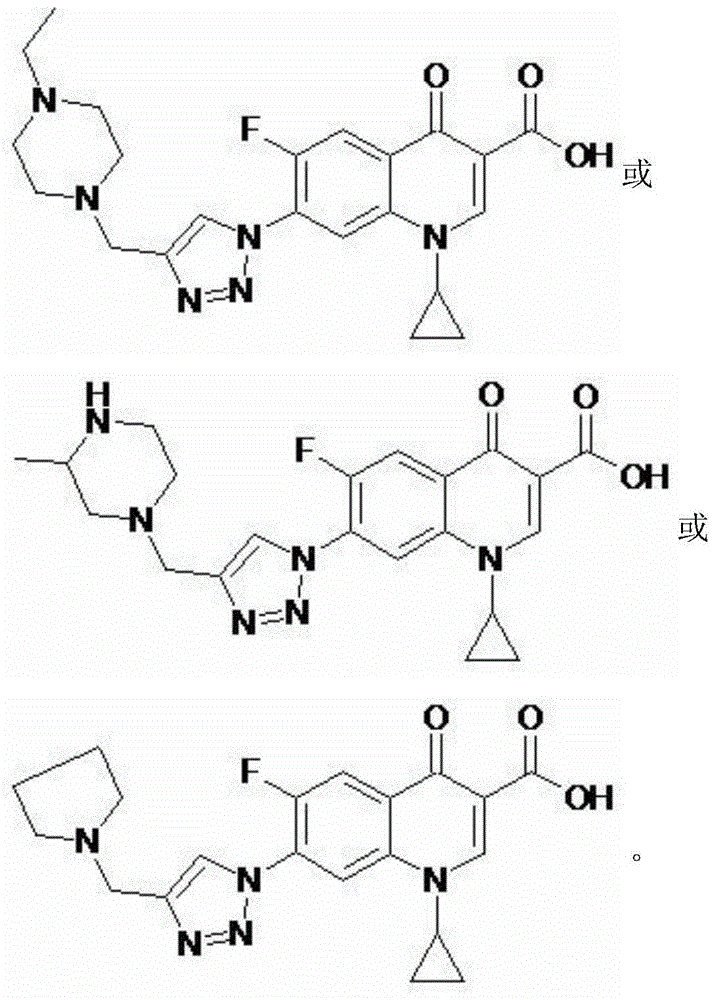
1-cyclopropyl-7-aminomethyl triazole-fluoroquinolones carboxylic acid derivative, method for preparing same and application of 1-cyclopropyl-7-aminomethyl triazole-fluoroquinolones carboxylic acid derivative
A technology of fluoroquinolone carboxylic acid and aminomethyl triazole, which is applied in the field of innovative drug synthesis to achieve the effect of overcoming drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 1-cyclopropyl-6-fluoro-7-(4-dimethylaminomethyl-[1,2,3]triazol-1-yl)-quinolin-4(1H)-one-3-carboxylic acid (I-1), its chemical structural formula is:
[0043]
[0044] That is, R in formula I is dimethylamino.
[0045] The preparation method of the compound is as follows: using dimethylamine as the amine donor, according to the above-mentioned general preparation method of the target object (I), the light yellow crystal (I-1) is obtained, the yield is 75.0%, m.p.226~228°C . 1 H NMR (400MHz, DMSO-d 6 ):δ15.46(1H,brs,COOH),8.88(1H,s,2-H),8.05(d,J=13.2Hz,1H,5-H),7.86~7.82(2H,m,5- H and 5″-H), 7.64 (1H, d, J=7.2Hz, 8-H), 5.35 (s, 2H, NCH 2 ),3.68~3.54(m,1H,1′-H),2.43(s,6H,2×CH 3 ), 1.27~1.18 (m, 4H, 2′- and 3′-H); MS(m / z): Calcd.for C 18 h 18 FN 5 o 3 :371.37[M] + ;Found:372[M+H] + .
Embodiment 2
[0047] 1-cyclopropyl-6-fluoro-7-(4-diethylaminomethyl-[1,2,3]triazol-1-yl)-quinolin-4(1H)-one-3-carboxylic acid (I-2), its chemical structural formula is:
[0048]
[0049] That is, R in formula I is diethylamino.
[0050] The preparation method of the compound is: using diethylamine as the amine donor, according to the above-mentioned general preparation method of the target object (I), to obtain a light yellow crystal (I-2), the yield is 64.0%, m.p.218~220°C . 1 H NMR (400MHz, DMSO-d 6 ):δ15.47(brs,1H,COOH),8.85(s,1H,2-H),8.03(d,J=13.2Hz,1H,5-H),7.86~7.76(2H,m,5- H and 5″-H), 7.62 (1H, d, J=7.2Hz, 8-H), 5.33 (2H, s, NCH 2 ),3.68~3.57(1H,m,1′-H),2.44~2.36(m,4H,2×CH 2 ),1.34~1.17(m,10H,2′-,3′-H and 2×CH 3 ); MS(m / z): Calcd.for C 20 h 22 FN 5 o 3 :399.43[M] + ;Found:400[M+H] + .
Embodiment 3
[0052] 1-cyclopropyl-6-fluoro-7-(4-piperidin-1-methyl-[1,2,3]triazol-1-yl)-quinolin-4(1H)-one-3- Carboxylic acid (I-3), its chemical structural formula is:
[0053]
[0054] That is, R in formula I is piperidin-1-yl.
[0055] The preparation method of the compound is as follows: using piperidine as the amine donor, according to the above-mentioned general preparation method of the target object (I), the light yellow crystal (I-3) was obtained with a yield of 63.0%, m.p.225-227°C. 1 H NMR (400MHz, DMSO-d 6 ):δ15.43(brs,1H,COOH),8.86(s,1H,2-H),8.07(d,J=13.2Hz,1H,5-H),7.87~7.82(2H,m,5- H and 5″-H), 7.65 (1H, d, J = 7.2Hz, 8-H), 5.31 (s, 2H, NCH 2 ),3.66~3.58(m,1H,1′-H),3.05~2.28(m,4H,N(CH 2 ) 2 ),1.63~1.08(m,10H,2′-,3′-H and 3×CH 2 ); MS(m / z): Calcd.for C 21 h 22 FN 5 o 3 :411.44[M] + ;Found:412[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com