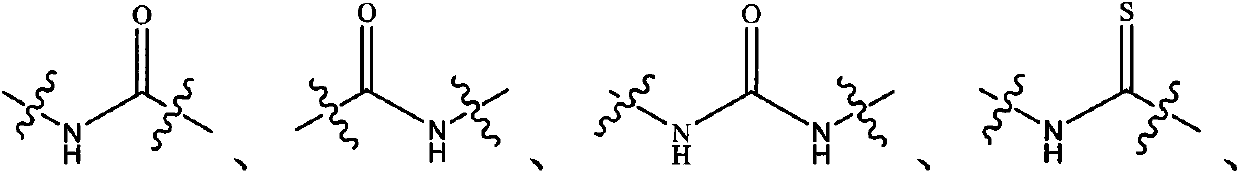
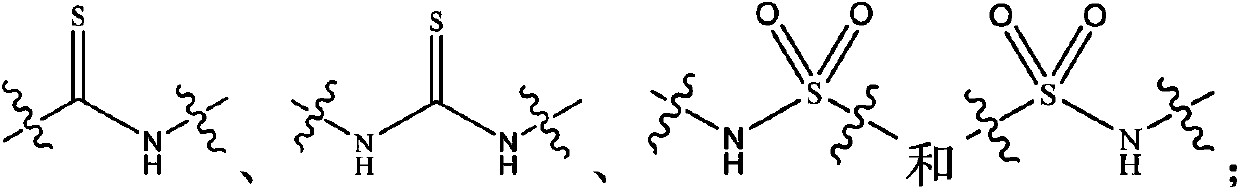
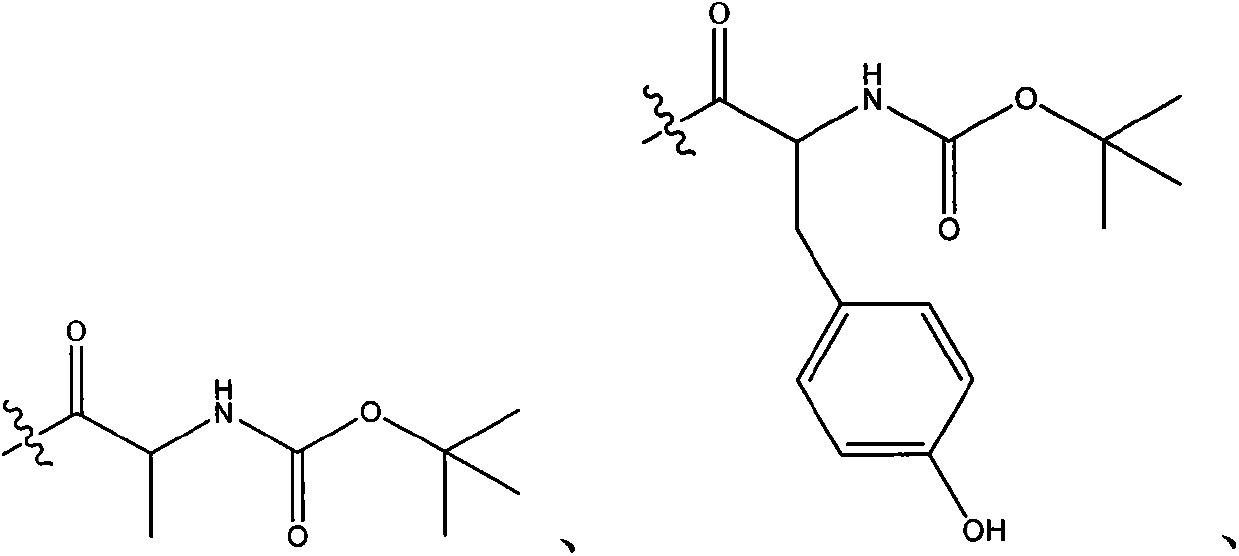
Kinase inhibitor with novel structure
A solvate, C1-C8 technology, applied in the field of kinase inhibitors with novel structures, can solve problems such as easy tolerability of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0093] Compound preparation
[0094] Compounds of formula (I) may be synthesized using standard synthetic techniques known to those skilled in the art or using methods known in the art in combination with the methods described herein. Additionally, solvents, temperatures and other reaction conditions given herein may be varied according to the skill in the art. As a further guide, the following synthetic methods can also be utilized.
[0095] The reactions can be used sequentially to provide the compounds described herein; or they can be used to synthesize fragments which are subsequently added by methods described herein and / or methods known in the art.
[0096] In certain embodiments, provided herein are methods of making and methods of using the tyrosine kinase inhibitor compounds described herein. In certain embodiments, the compounds described herein can be synthesized using the following synthetic schemes. Compounds can be synthesized using methods similar to those ...
Embodiment 1
[0148] Synthesis of (4-methyl-3-(N-(4(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)-4-(propynamidomethyl)benzamide compound 1
[0149] With 0.05mmol 4-(methyl)-N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)benzamide k reaction temperature down Zero degrees Celsius, 0.05mmol of N-(4-methyl-3-(piperidine-4-oxyl)phenyl)-4-((4-methylpiperazin-1-yl)methyl)-3- (Trifluoromethyl)benzamide trihydrochloride h, 0.05mmol propiolic acid, 0.3mmol N,N-diisopropylethylamine (DIPEA) and 1 ml N,N-dimethylformamide (DMF ) into a 5-milliliter round-bottomed flask successively, and 0.06 mmol of 2-(7-azobenzotriazole)-N, N, N', N'-tetramethyluronium hexafluorophosphate ( HATU). The reaction system was stirred at room temperature for 2 hours. The reaction system was extracted with ethyl acetate, dried over anhydrous sodium sulfate; the solvent was removed by a rotary evaporator, and the crude product was separated by a silica gel column to obtain the target compound 1 (yield: 38%). Exa...
Embodiment 2
[0151] (E)-(4-(4-(Dimethylamino)-2-acrylamide)methyl)-(4-methyl-3-(4-(3-pyridyl)pyrimidin-2-yl)amino) Synthesis of phenyl)benzamide compound 2
[0152] Synthesis of Compound 2 was accomplished using a procedure similar to that described in Example 1. Exact Mass (calculated value): 521.25; MS (ESI) m / z (M+1) + : 522.27.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com