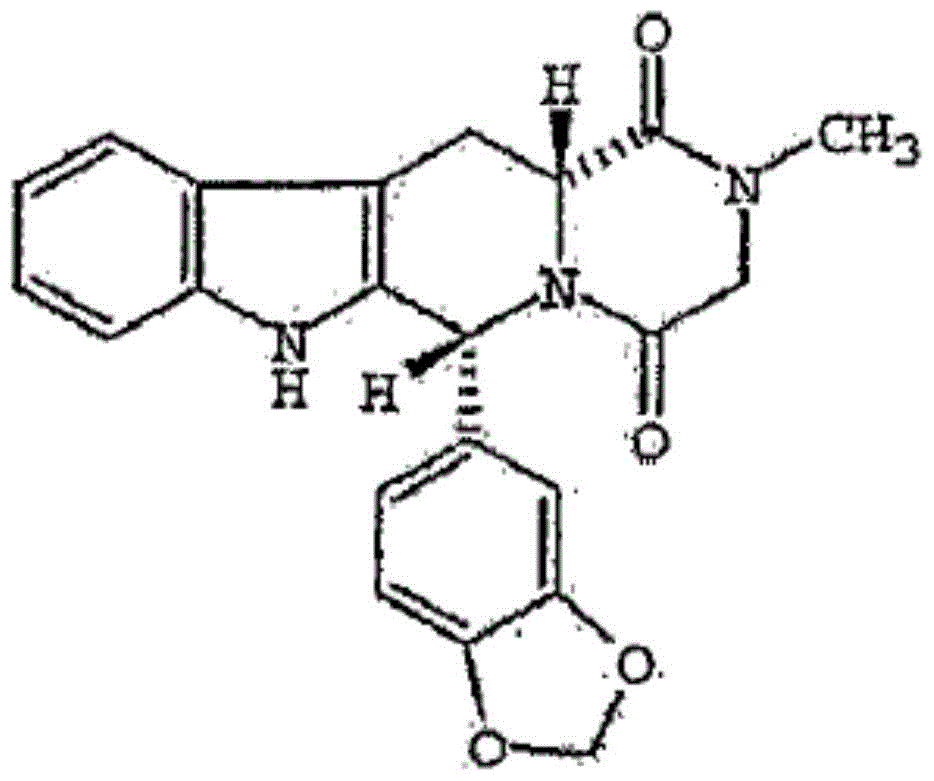
A kind of tadalafil compound, and composition thereof
A technology of tadalafil and composition, applied in the field of tadalafil compounds, capable of solving problems such as improvement of dissolution time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Preparation of tadalafil hydrate crystals:
[0096] (1) Dissolve 10g of tadalafil in 100mL of a mixed solvent of acetone, dimethylformamide and water at a temperature of 40°C to obtain solution A. In the mixed solvent, acetone, dimethylformamide and The volume ratio of water is 8.0:3.8:1;
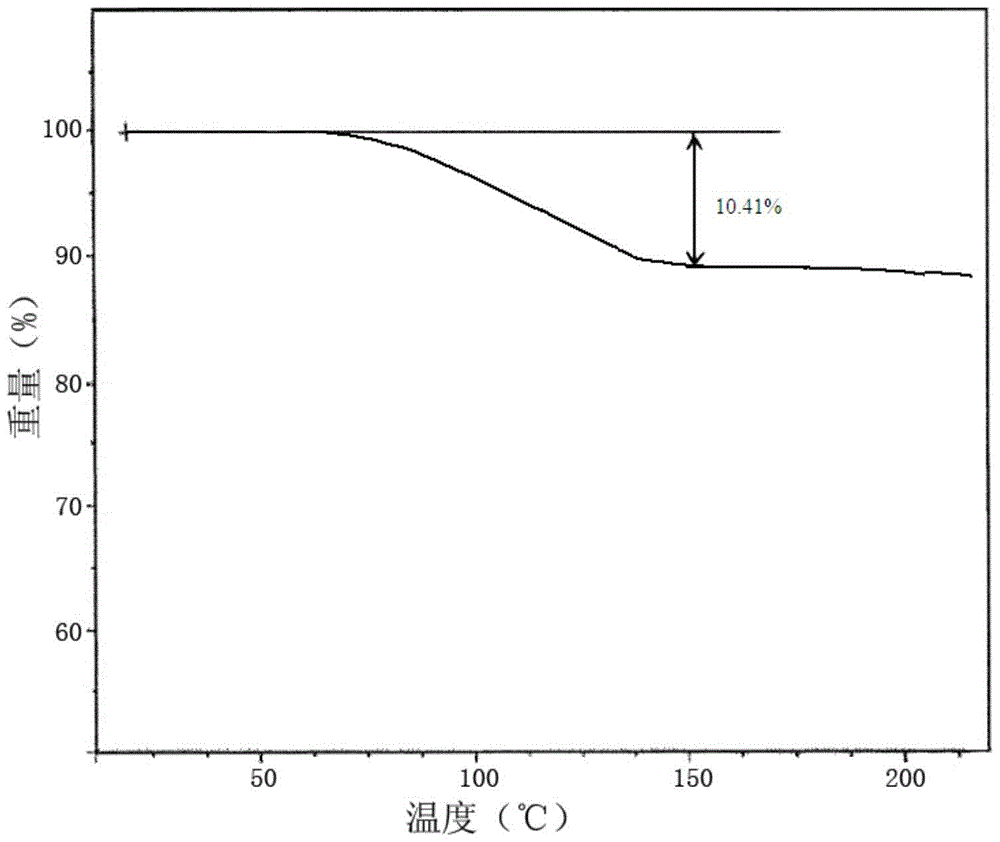
[0097] (2) Add anhydrous diethyl ether to solution A. The volume of anhydrous diethyl ether added is twice the volume of solution A. Under the condition of stirring, the temperature is lowered to -5°C within 2.5 hours, and the stirring speed is controlled at 400r / min, and then placed at -5°C for 8.0 hours, crystals were precipitated, filtered, washed with anhydrous ether, and dried to obtain tadalafil crystals containing 2.5 crystal waters.
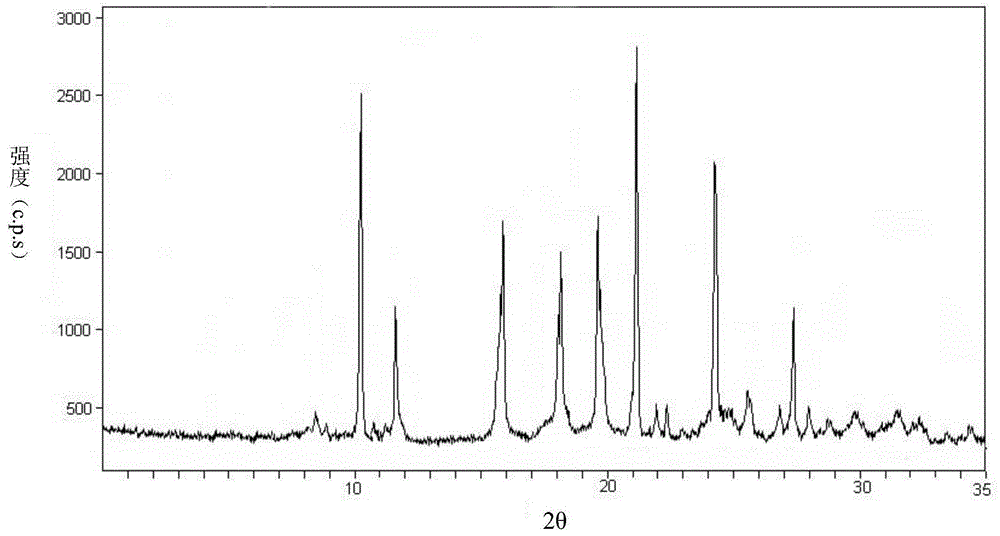
[0098] The obtained product tadalamorphic compound adopts Cu-Kα 1 X-ray powder diffraction for radiographic measurements such as figure 1 Shown, in (2θ±0.1) be 10.2 °, 11.6 °, 15.9 °, 18.0 °, 19.4 °, 21.0 °, 24.2 °, 27.2 ° place shows charac...
Embodiment 2
[0100] Preparation of tadalafil hydrate crystals:
[0101] (1) Dissolve 10g of tadalafil in 100mL of a mixed solvent of acetone, dimethylformamide and water at a temperature of 30°C to obtain solution A. In the mixed solvent, acetone, dimethylformamide and The volume ratio of water is 6.8:3.0:1;
[0102] (2) Add anhydrous diethyl ether to solution A, the volume of anhydrous diethyl ether is 1.5 times the volume of solution A, under the condition of stirring, lower the temperature to 0°C within 3 hours, and control the stirring speed at 450r / min , and then placed at 0°C for 8 hours, crystals were precipitated, filtered, washed with anhydrous ether, and dried to obtain tadalamorph crystals containing 2.5 crystal water. The obtained product tadalamorphic compound adopts Cu-Kα 1 The X-ray powder diffraction analysis of X-ray measurement, and thermogravimetric (TG) analysis, its result is consistent with embodiment 1.
Embodiment 3
[0104] Preparation of tadalafil hydrate crystals:
[0105] (1) Dissolve 10 g of tadalafil in 100 mL of a mixed solvent of acetone, dimethylformamide and water at a temperature of 35° C. to obtain solution A. In the mixed solvent, acetone, dimethylformamide The volume ratio of amides and water is 8.0:4.5:1;
[0106] (2) Add anhydrous diethyl ether to solution A. The volume of anhydrous diethyl ether added is 1.5 times the volume of solution A. Under the condition of stirring, the temperature is lowered to -3°C within 2.5 hours, and the stirring speed is controlled at 450r / min, and then placed at -5°C for 10 hours, crystals were precipitated, filtered, washed with anhydrous ether, and dried to obtain tadalamorph crystals containing 2.5 crystal waters. The obtained product tadalamorphic compound adopts Cu-Kα 1 The X-ray powder diffraction analysis of X-ray measurement, and thermogravimetric (TG) analysis, its result is consistent with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com