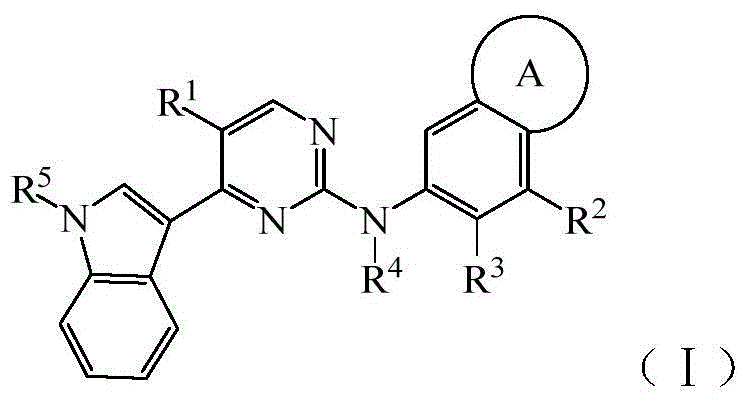
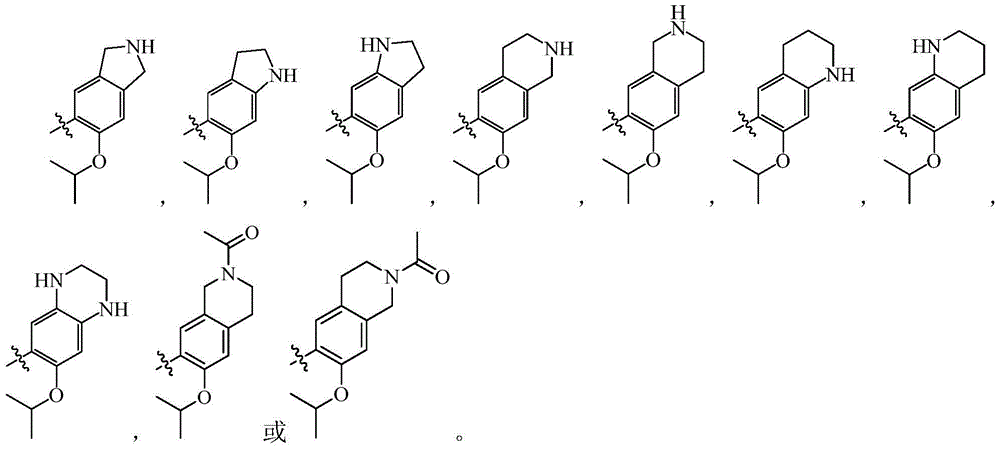
Pyrimidine Derivatives Anaplastic Lymphoma Kinase Inhibitors
A technology of immunosuppressants and drugs, applied to esters or solvates, can solve problems such as easy drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0076] Step 1 Preparation of Intermediate 1
[0077] Dissolve raw material 1 in a solvent (such as N,N-dimethylformamide), add potassium hydroxide and iodine, stir (for example, 30 minutes), then add potassium hydroxide and p-toluenesulfonyl chloride, and stir at room temperature (such as 2 hours), quenched by adding water, and obtained intermediate 1 by suction filtration.
[0078] Step 2 Preparation of Intermediate 2
[0079] Intermediate 1 is dissolved in a solvent (such as dioxane), and 4,4,5,5-tetramethyl-1,3,2-dioxaborolane and a suitable palladium catalyst (such as tetrathree Phenylphosphine palladium) is added with a base (such as triethylamine), heated (such as 80° C.) overnight under nitrogen protection, cooled (such as room temperature), quenched with water, extracted with an organic solvent (such as ethyl acetate), concentrated, and purified ( Such as silica gel column chromatography) to obtain intermediate 2.
[0080] Step 3 Preparation of Intermediate 3
[00...
experiment example 1
[0142] Experimental example 1 In vitro enzymatic activity test of the compound of the present invention
[0143] Test product: trifluoroacetate salt of compound 1, compound 2 and compound 4 of the present invention. For their chemical names and preparation methods, see the preparation examples of trifluoroacetate salt of compound 1, compound 2 and compound 4.
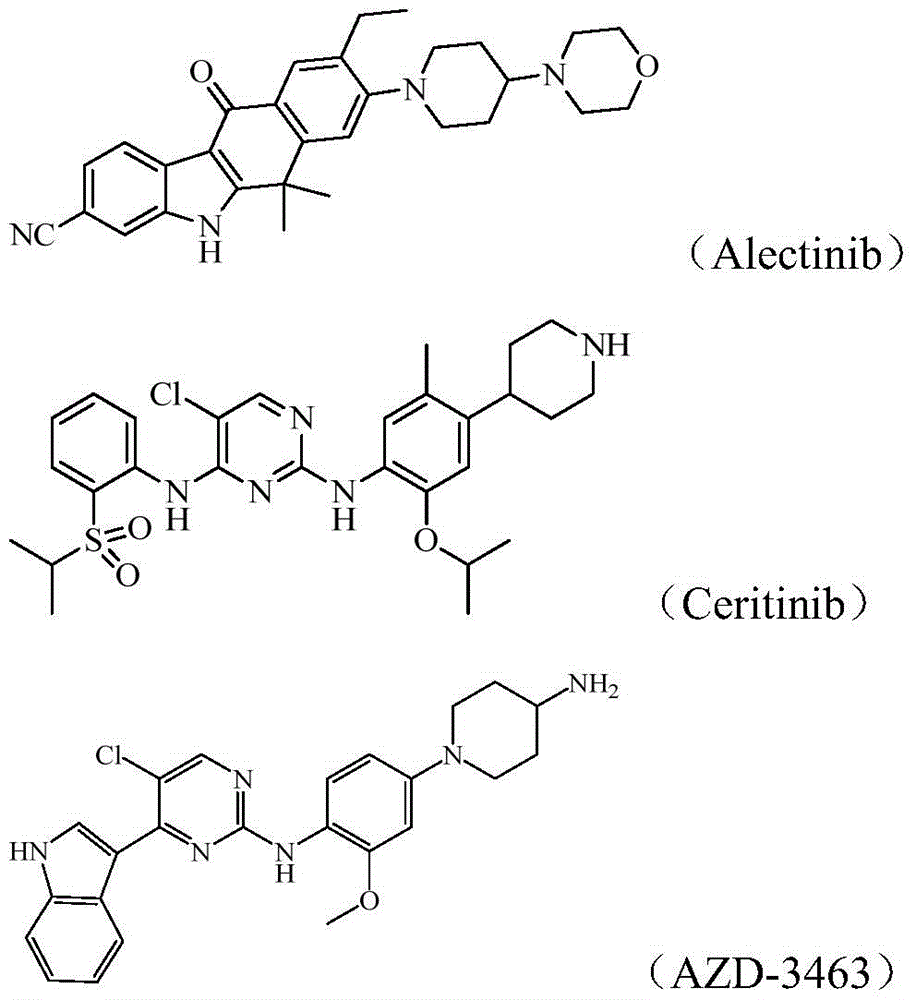
[0144] The control drug ceritinib is self-made (refer to the preparation method of patent WO2008 / 073687A2).
[0145] The meanings represented by the abbreviations of the following experiments are as follows:
[0146] DMSO: dimethyl sulfoxide
[0147] DTT: Dithiothreitol
[0148] SEB: Enzyme Catalyst Buffer
[0149] ATP: adenosine triphosphate
[0150] ALK: Anaplastic Lymphoma Kinase
[0151] SA-XL665: Streptavidin-labeled donor
[0152] 2.5×, 5×, 10× “×” among them: times
[0153] experimental method:
[0154] ALK kinase buffer preparation:
[0155] Take an appropriate amount of MgCl2, 2500nM SEB, 100mM DTT, a...
experiment example 2
[0179] Experimental example 2 In vitro cell activity test of the compound of the present invention
[0180] Test product: trifluoroacetate salt of compound 1 and compound 2 of the present invention. For their chemical names and preparation methods, see the preparation examples of trifluoroacetate salt of compound 1 and compound 2.
[0181] The control drug 1 Ceritinib is self-made (prepared with reference to the preparation method of patent WO2008 / 073687A2), and its structural formula is as described in the background technology.
[0182] The control drug 2 Alectinib is self-made (prepared with reference to the preparation method of patent CN102459172A), and its structural formula is as described in the background technology.
[0183] The meanings represented by the abbreviations of the following experiments are as follows:
[0184] rpm: revolutions per minute
[0185] DMSO: dimethyl sulfoxide
[0186] MTS: Thiazolium blue tetrazolium bromide
[0187] RPMI1640: 1640 medi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com