Living lung slicing based anti-influenza virus or anti-inflammatory drug screening model establishing method and application
A technology of anti-influenza virus and anti-inflammatory drugs, applied in the field of medicine, can solve the problems of inaccurate evaluation, no drug screening, no development of drug evaluation models, etc., and achieve the effect of saving experimental materials and saving usage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1 Preparation of lung slices
[0059] (1) After the mice were anesthetized by intraperitoneal injection of pentobarbital (75 mg / kg), they were sacrificed by exsanguination from the abdominal aorta. The abdominal skin was wiped and disinfected with alcohol cotton balls. After the trachea was exposed, the trachea was separated with ophthalmic forceps, and a "V"-shaped incision was made at the trachea (centrifugal end) with ophthalmic scissors. Insert an 18-gauge ground syringe needle through the "V" incision and secure with suture. Then fill the lungs with 1.3ml of low-melting gel preheated to 37°C.
[0060] (2) Place the whole mouse on ice for 10 minutes to allow the gel to initially solidify. After the gel solidified, cut off the trachea, carefully remove the heart and the whole lung, put them in pre-cooled DMEM / F12 medium, and place them at 4°C for 15 minutes to make the gel completely solidify. Separate the lung and select the left lung lobe. Use ophthalm...
Embodiment 2
[0063] Embodiment 2 The thickness of lung section is preferred
[0064] (1) with 10 5 H1N1 virus infection of PFU / ml Lung slice sample 1, sample 2, sample 3 and sample 4 prepared in Example 1. After 2 hours of infection, the virus solution was discarded, washed twice with phosphate buffer, and fresh culture solution was added.
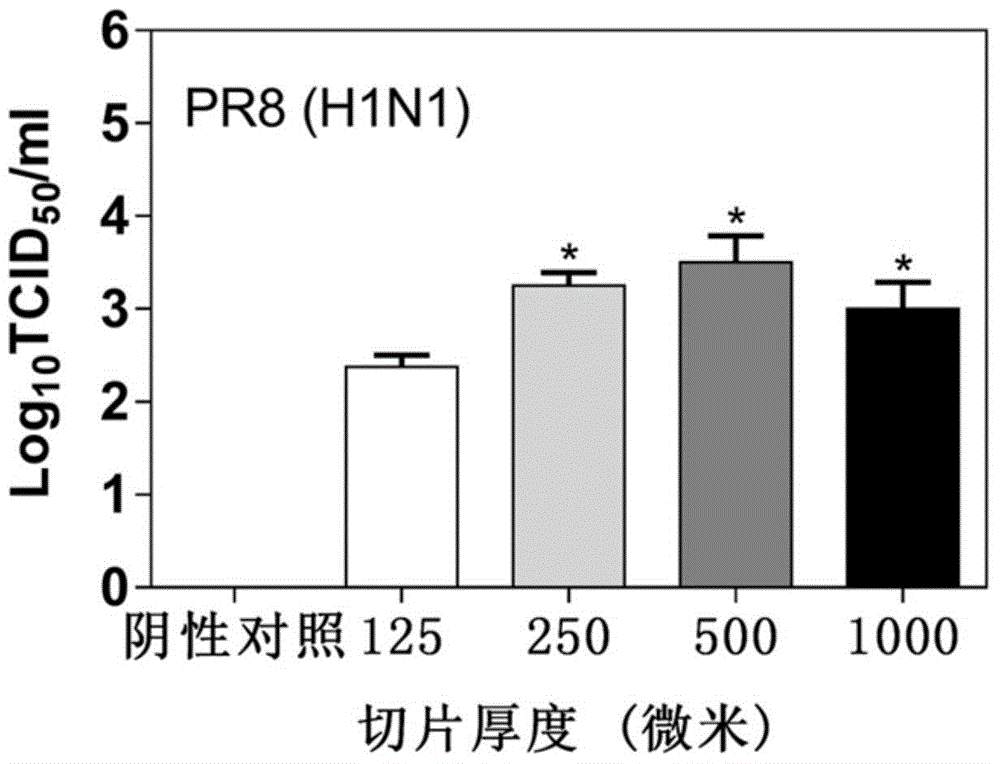
[0065] (2) Collect the supernatant after 48 hours. Tissue median infectious dose (TCID) 50 ) to detect the virus titer in the section supernatant, and the virus titer was calculated using the Reed–Muench method (Reed, L.J., Muench, H., 1938. American Journal of Epidemiology 27, 493-497.). The result is as figure 1 As shown, it can be seen that the log in samples 1-4 10 The TCID50 values were 2.375, 3.25, 3.5, and 3, respectively. The virus particles in the supernatant of sample 2 were 7.5 times more than those in the lung slice supernatant of sample 1, but the difference was not obvious from sample 3 and sample 4. In sample 2-sample 4, the thic...
Embodiment 3
[0066] Example 3 Neuraminidase (NA) activity and viral activity correlation evaluation
[0067] (1) with 10 5 PFU / ml H1N1 virus or H3N2 virus infection lung slice sample 2 prepared in Example 1. After 2 hours of infection, the virus solution was discarded, washed twice with phosphate buffer, and fresh culture solution was added.
[0068] (2) The supernatants of lung slice samples were collected after 12 hours, 24 hours, 48 hours, 72 hours, 96 hours and 120 hours.
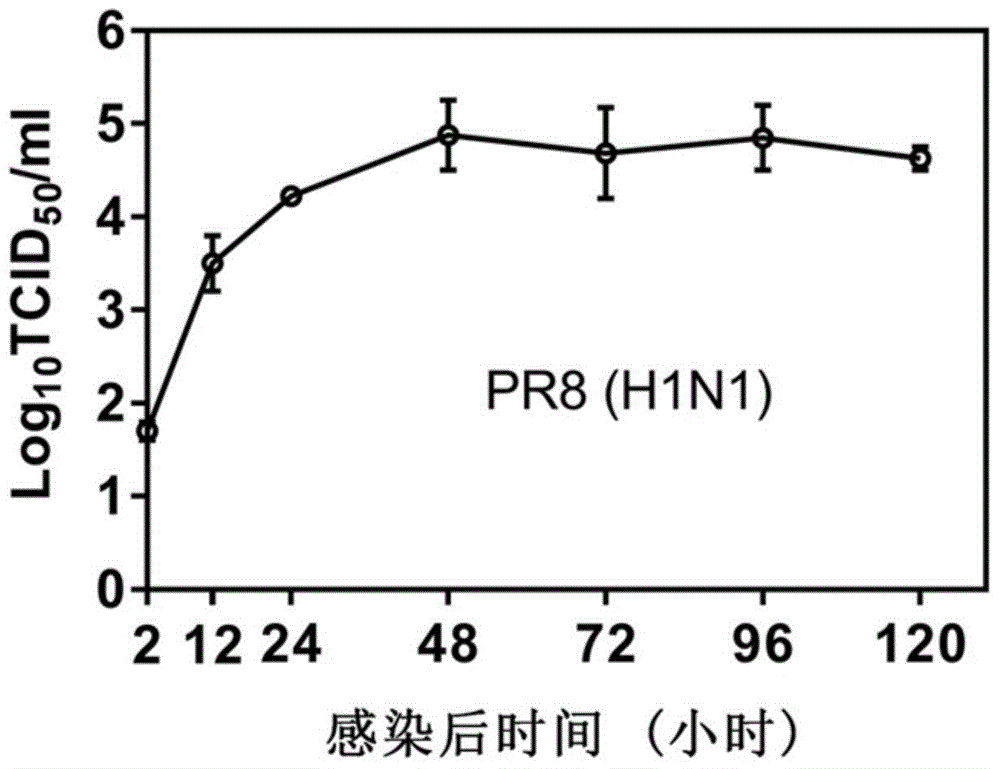
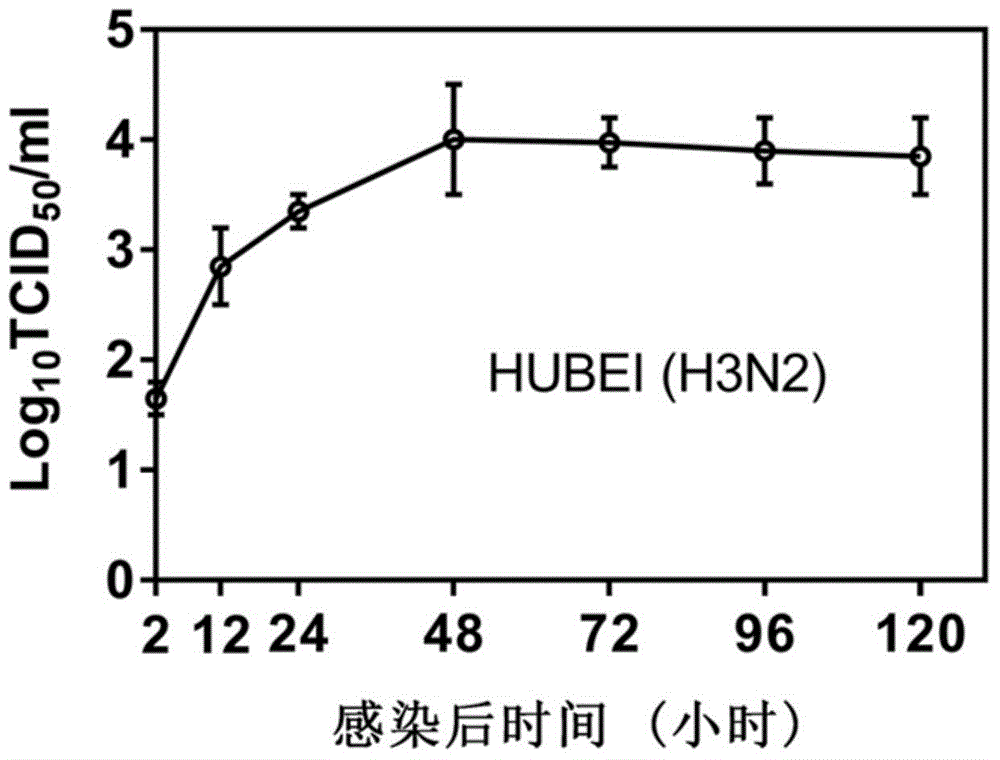
[0069] (3) The virus titer in the supernatant collected in step (2) was detected by tissue half infectious dose (TCID50), and the virus titer was calculated by the Reed-Muench method. The result is as Figure 2a , 2b As shown, the virus reached the replication peak at 48h and entered the stable replication stage.
[0070] (4) 2'-(4-methylumbelliferone)-α-D-acetylneuraminic acid (MUNANA) is a specific fluorescent substrate for neuraminidase (NA), used to express neuraminidase active. Put 40 μl of the virus s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com