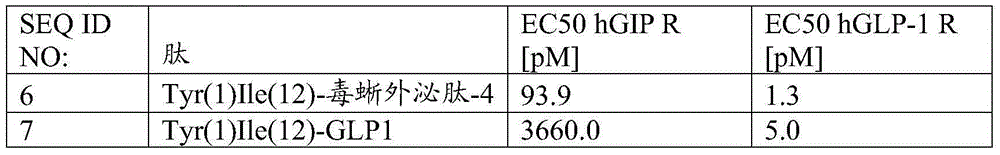
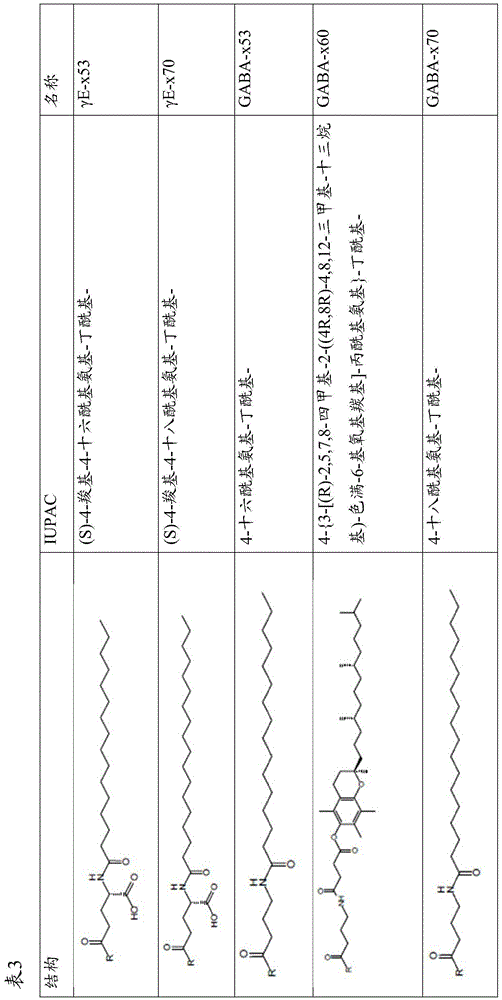
Exendin-4 derivatives as dual GLP1/GIP or trigonal GLP1/GIP/glucagon agonists
A technology of peptide compounds and solvates, applied in glucagon, hormone peptides, antidote, etc., can solve the problems of chemical instability of exendin-4
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0597] Synthesis of SEQ ID NO:12
[0598]In Novabiochem Rink-Amide resin (4-(2',4'-dimethoxyphenyl-Fmoc-aminomethyl)-phenoxyacetamido-norleucylaminomethyl resin), 100-200 The solid-phase synthesis was carried out on a mesh with a loading of 0.34 mmol / g. The Fmoc-synthesis strategy was applied with HBTU / DIPEA-activation. Boc-Tyr(tBu)-OH at position 1 and Fmoc-Lys(ivDde)-OH at position 14 were used in the solid phase synthesis protocol. The ivDde-group was cleaved from the peptide on the resin with 4% hydrazine hydrate in DMF according to a modified literature procedure (S.R. Chhabra et al., Tetrahedron Lett. 39, (1998), 1603). Thereafter Palm-Glu(γОSu)-OtBu was coupled to the detethered amino group. Peptides were cleaved from the resin with King's mixture (D.S. King, C.G. Fields, G.B. Fields, Int. J. Peptide Protein Res. 36, 1990, 255-266). The crude product was purified by preparative HPLC on a Waters column (Sunfire, Prep C18) with an acetonitrile / water gradient, both buf...
Embodiment 2
[0600] Synthesis of SEQ ID NO:31
[0601] In Novabiochem Rink-Amide resin (4-(2',4'-dimethoxyphenyl-Fmoc-aminomethyl)-phenoxyacetamido-norleucylaminomethyl resin), 100-200 The solid phase synthesis was carried out on the loading amount of 0.34mmol / g. The Fmoc-synthesis strategy was applied with HBTU / DIPEA-activation. Boc-Tyr(tBu)-OH at position 1 and Fmoc-Lys(ivDde)-OH at position 14 were used in the solid phase synthesis protocol. The ivDde-group was cleaved from the peptide on the resin with 4% hydrazine hydrate in DMF according to a modified literature procedure (S.R. Chhabra et al., Tetrahedron Lett. 39, (1998), 1603). Thereafter Stea-Glu(γОSu)-OtBu was coupled to the detethered amino group. Peptides were cleaved from the resin with King's mixture (D.S. King, C.G. Fields, G.B. Fields, Int. J. Peptide Protein Res. 36, 1990, 255-266). The crude product was purified by preparative HPLC on a Waters column (Sunfire, Prep C18) with an acetonitrile / water gradient, both buffer...
Embodiment 3
[0603] Synthesis of SEQ ID NO:14
[0604] Solid phase synthesis was performed on Rink-resin from Agilent Technologies with a loading of 0.38 mmol / g, 75-150 μm. The Fmoc-synthesis strategy was applied with HBTU / DIPEA-activation. Boc-Tyr(tBu)-OH at position 1 and Fmoc-Lys(ivDde)-OH at position 14 were used in the solid phase synthesis protocol. The ivDde-group was cleaved from the peptide on the resin according to the literature (S.R. Chhabra et al., Tetrahedron Lett. 39, (1998), 1603). This was followed by coupling of Palm-Glu(γОSu)-OtBu to the detethered amino group using HBTU / DIPEA coupling reagent, followed by Fmoc-deprotection with 20% piperidine in DMF. Peptides were cleaved from the resin using King's mixture (D.S. King, C.G. Fields, G.B. Fields, Int. J. Peptide Protein Res. 36, 1990, 255-266). The crude product was purified by preparative HPLC on a Waters column (XBridge, BEH130, Prep C18 5 μΜ) with an acetonitrile / water gradient, both buffered with 0.1% TFA. The pur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com