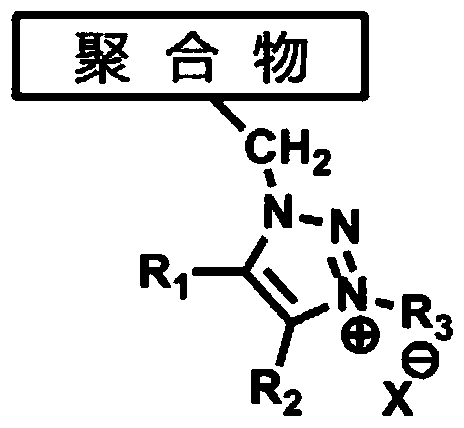
A kind of 1,2,3-triazole salt polymer and its preparation method and application
A technology of triazole salts and polymers, which is applied in the field of polymers and preparation of 1,2,3-triazole salts, can solve the problems of poor alkaline stability, low anion conductivity, unusability, etc. High stability, wide source of raw materials and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
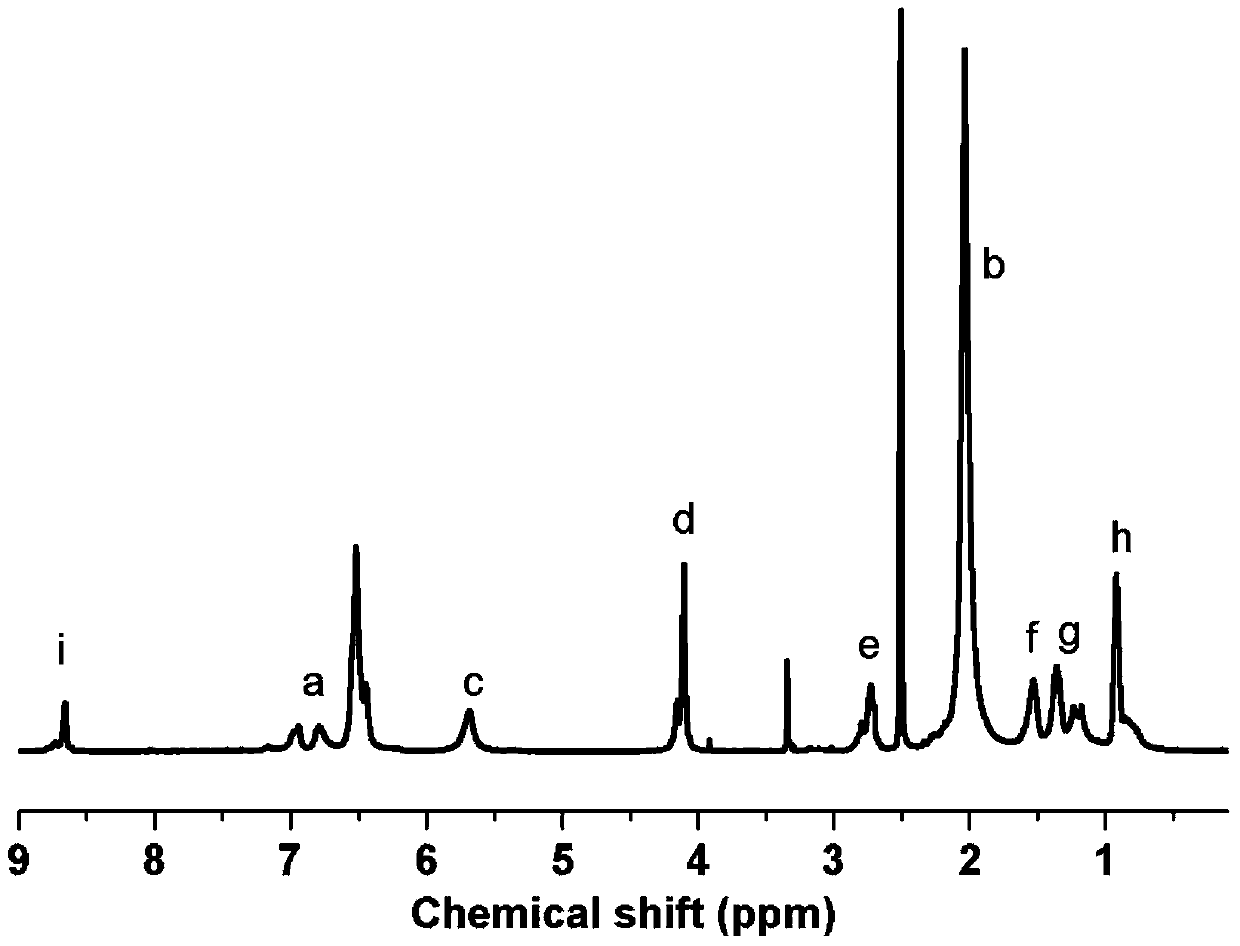
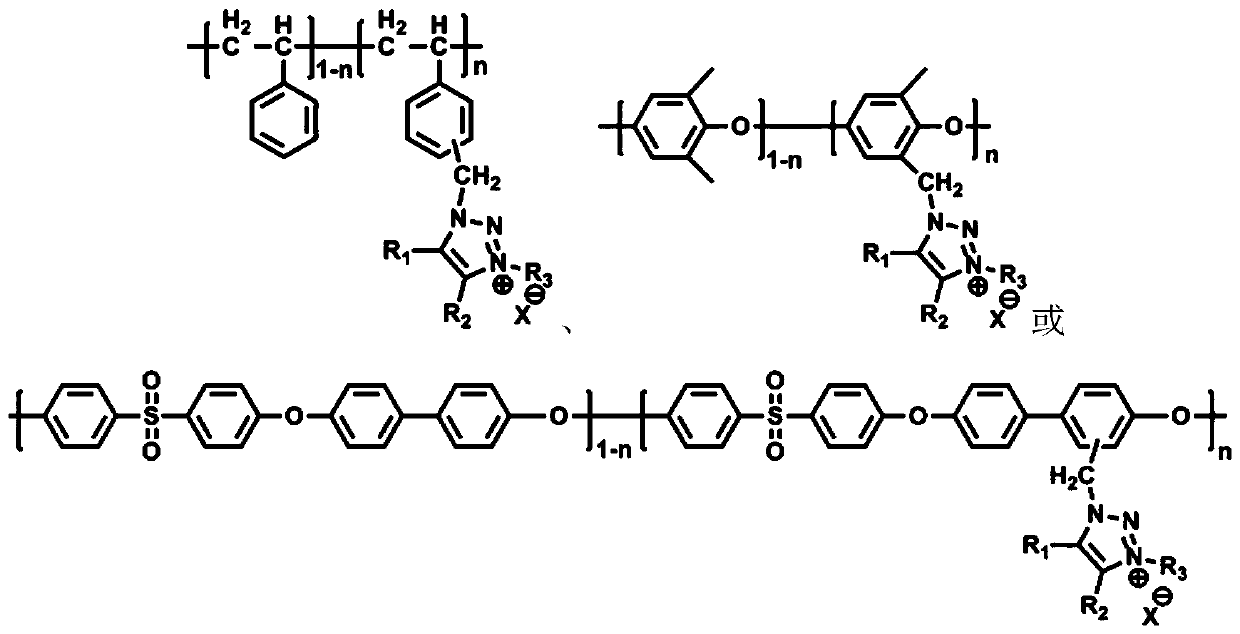
[0038] Dissolve 2.136g of brominated polyphenylene ether (the degree of bromination is 27%) in 20mL of N-methylpyrrolidone, stir magnetically to form a homogeneous solution, add 1.325g of sodium azide, and react at 60°C for 12 hours. Precipitate in methanol / water (v / v=5 / 1) mixed solution, wash 4 times, filter and dry to obtain methyl azidated polyphenylene ether.
[0039] Add 1.000g of the prepared methyl azide polyphenylene ether, 0.34mL of 1‐hexyne, 0.1076g of cuprous bromide, and 0.313mL of N,N,N',N,'N into a 25mL Schlenk reactor. ‐pentamethyldiethylenetriamine and 7mL N‐methylpyrrolidone, and the reactor was sealed, and immersed in liquid nitrogen, after 3 times of vacuum ‐ nitrogen ‐thawing, the oxygen in the reactor was excluded. After the reactor was placed in an oil bath at 60°C for 6 hours, the solution was precipitated in a large amount of methanol, washed, filtered, and dried to obtain 1,2,3‐triazole polyphenylene ether.
[0040] Dissolve 0.857g of the prepared 1,2...
Embodiment 2
[0044] Dissolve 2.000g of chloromethylated polyethersulfone (chloromethylation content: 16%) in 20mL of N,N-dimethylformamide, stir magnetically to form a homogeneous solution, then add 0.231g of sodium azide , after reacting at 80°C for 8 hours, precipitate in methanol / water (v / v=1 / 1) mixed solution, wash 5 times, filter and dry to obtain methyl azide polyethersulfone.
[0045]Add 1.000g of prepared methyl azide polyethersulfone, 0.046mL of phenylacetylene, 0.0152g of cuprous bromide, 0.044mL of N,N,N',N,'N"- Pentamethyldiethylenetriamine and 7mL N,N-dimethylformamide, and the reactor was sealed and immersed in liquid nitrogen. After 3 times of vacuum-nitrogen-thawing, the oxygen in the reactor was Exclusion. After putting the reactor into an oil bath at 60°C for 8 hours, the solution was precipitated in a large amount of methanol, washed, filtered and dried to obtain 1,2,3-triazole polyethersulfone.
[0046] Dissolve 0.800g of the prepared 1,2,3-triazole polyethersulfone an...
Embodiment 3
[0050] Dissolve 4.000g of a copolymer of p-chloromethylstyrene and styrene (the molar content of p-chloromethylstyrene is 30%) in 20mL of N, N-dimethylacetamide, magnetically stirred to form a homogeneous solution, Add 3.286g of sodium azide, react at 70°C for 8 hours, precipitate in methanol / water (v / v=1 / 1) mixed solution, wash twice, filter and dry to obtain methyl azide polystyrene.
[0051] In a 25mL Schlenk reactor, add 2.00g of prepared methyl azide polystyrene, 0.516mL of 1‐hexyne, 0.162g of cuprous bromide, 0.472mL of N,N,N',N,'N "-pentamethyldiethylenetriamine and 7mL N,N-dimethylacetamide, and the reactor was sealed and immersed in liquid nitrogen. Oxygen was excluded. After putting the reactor into a 70°C oil bath for 6 hours, the solution was precipitated in a large amount of methanol, washed, filtered, and dried to obtain 1,2,3‐triazole polystyrene.
[0052] Dissolve 1.50 g of prepared 1,2,3-triazole polystyrene and 1.76 mL of 1-iodobutane in 8 mL of N,N-dimethy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water absorption | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com