Pharmaceutical composition containing solifenacin succinate
A technology of solifenacin succinate and a composition, which can be applied in the directions of drug combinations, active ingredients of heterocyclic compounds, and medical preparations of non-active ingredients, etc., can solve the problems of poor dissolution of solifenacin succinate tablets and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
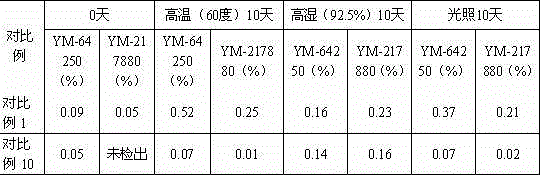
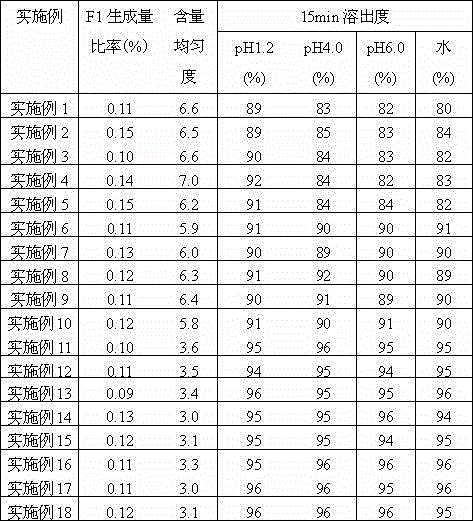
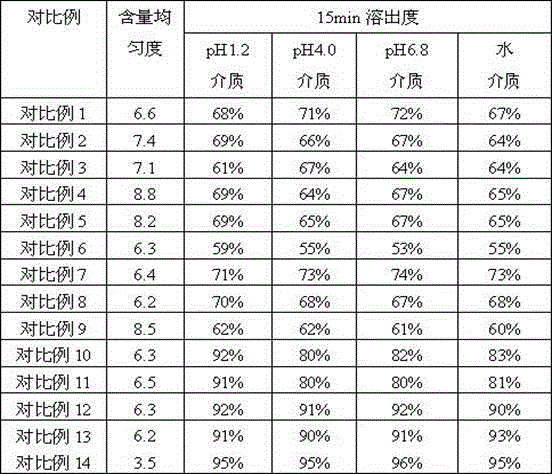
Image
Examples
Embodiment Construction
[0105] Below in conjunction with specific embodiment, further illustrate the present invention. But these examples are only limited to illustrate the present invention rather than further limiting the protection scope of the present invention.
[0106] composition Content (mg / tablet) weight percentage Solifenacin Succinate 4.5 1.5wt% Direct Compressed Lactose Tablettose 70 270 90wt% Sodium carboxymethyl starch 3.5 1.17wt% Highly substituted hydroxypropyl cellulose 15 5.33wt% Micropowder silica gel 6 2wt%
[0107] Weigh the required raw and auxiliary materials according to the prescription amount, first mix solifenacin succinate with sodium carboxymethyl starch and high-substituted hydroxypropyl cellulose, then add 10% direct compression lactose, and add 20% of Directly compress lactose, mix evenly, then add 30% direct-compressed lactose for mixing, add the remaining direct-compressed lactose, mix and pass through a 50-me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com