Solifenacin succinate new preparation method
A technology of solifenacin succinate and succinic acid, which is applied in the field of preparation of raw materials, can solve the problems of instability of raw material formula X compound and unsuitability for industrial production, etc., and achieves the advantages of easy operation, simple preparation process and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
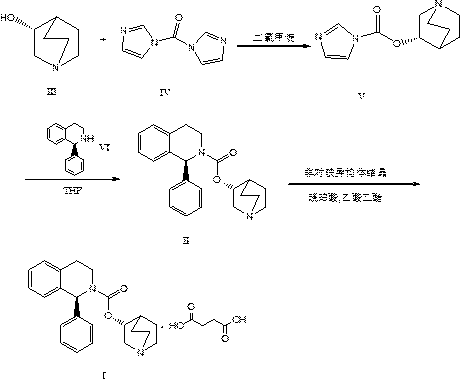
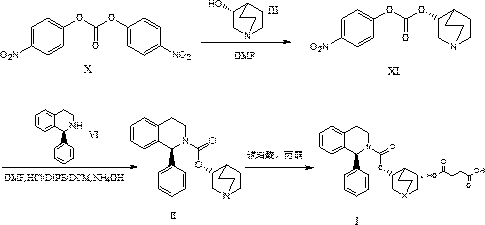
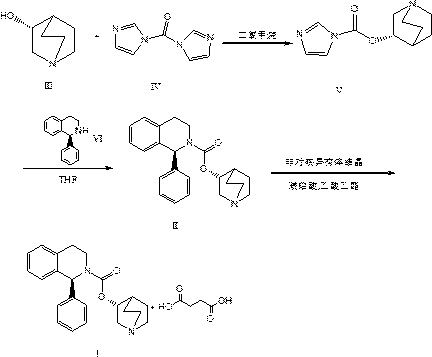
[0030] 1) At 0°C, suspend 127.2g of formula III compound R)-(-)-quinuclidin-3-ol in 2L of dichloromethane, and add 194.6g of formula IV compound DCI; then, under inert gas Stir the reaction for 3 hours; after the reaction, add water and extract, the organic layer is dried with anhydrous sodium sulfate, and finally dichloromethane is distilled off under reduced pressure, and crystallized with isopropyl acetate (IPAC)-heptane to obtain 164.8 g of the compound of formula V , as a white solid with a yield of 85.4%.
[0031] 2) Under inert gas, cool the tetrahydrofuran solution to -10°C, add 148.9g of formula VI compound (S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline, and then slowly add 164.8g of the compound of formula V was stirred and reacted for 2h; the temperature was raised to room temperature, water was added and extracted with tert-butyl methyl ether, the organic phase was dried over anhydrous sodium sulfate, and tetrahydrofuran was distilled off under reduced pressure to obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com