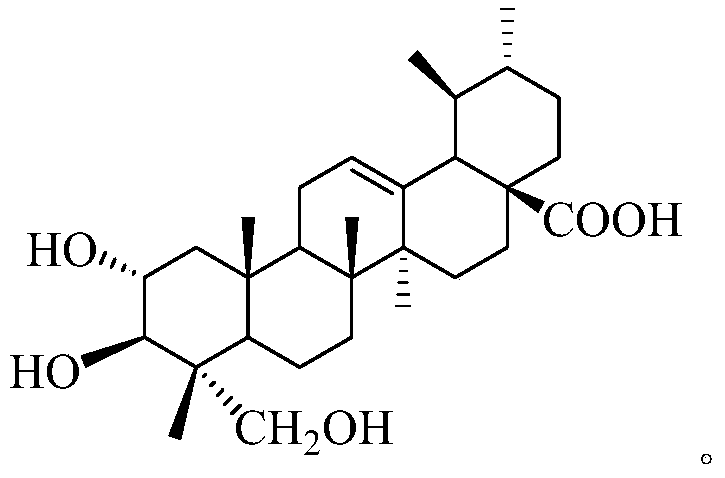
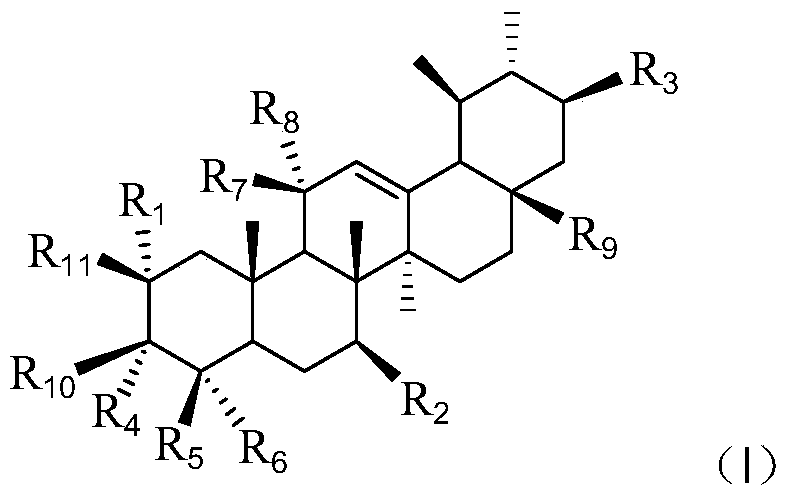
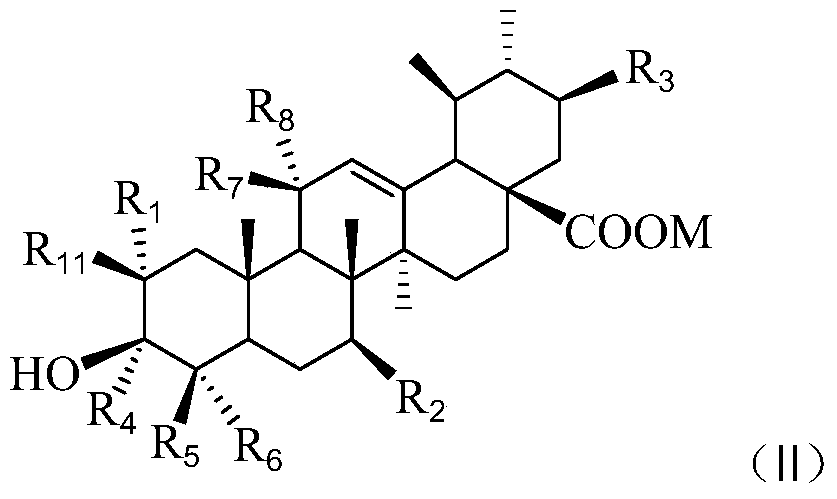
Asiatic acid derivatives, their preparation methods and their application in the preparation of antidepressants
An antidepressant and drug technology, applied in the field of natural medicinal chemistry, can solve the problems of application limitations, large adverse reactions, poor bioavailability, etc., and achieve high antidepressant activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Preparation and extraction of compound 1:
[0049] Inoculate Gliocladium roseum CGMCC 3.3657 strains of Gliocladium roseum CGMCC 3.3657 with 2% inoculum in 10 bottles of 1000ml (400ml medium per bottle), place in a constant temperature shaker, 180rpm, 27°C for 72 hours, then add 300mg Substrate asiatic acid (dissolved in 20ml of ethanol, 2ml added to each bottle), continued to cultivate for 10 days. After the fermentation is completed, filter the mycelium, extract the filtrate 3 times with equal volumes of ethyl acetate, and extract the mycelia three times with 500ml ethyl acetate ultrasonically, each time for 30min, combine the extract and the extract, and extract the ethyl acetate extract Concentrate to a small volume on a rotary evaporator and dry to obtain conversion reaction extracts (1.8 g).
[0050] Dissolve the conversion reaction extract with a small amount of ethanol, mix the sample with three times the weight of silica gel, separate on 25g of silica gel, and...
Embodiment 2
[0055] Structural identification of compound 1:
[0056] Compound 1 is a white solid, HR-ESI-MS gives 501.3243 ([M-H]-), combined with 1H-NMR and 13C-NMR to deduce that the molecular formula is C30H46O6. In 1H-NMR (600MHz, pyridine-d5), it shows that the compound There are six groups of methyl hydrogen signals δ1.39(3H,o), δ1.17(3H,s), δ1.08(3H,d,6.6), δ1.03(3H,s), δ0.95(3H ,s), δ0.83(3H,s); there is an active hydrogen signal at the downfield: δ5.50(t,3.6), which may be the hydrogen on the double bond. 13C-NMR shows two unsaturated carbon atoms δ125.9 and δ139.8, one carboxyl carbon atom δ179.5. Based on the above information, it can be inferred that the compound is a ursane-type pentacyclic triterpene acid compound.
[0057] According to HSQC and HMBC spectra, the hydrocarbons of compound 1 can be further assigned.
[0058] Compared with asiatic acid, in 1H-NMR spectrum, compound 1 has a hydrogen signal at 3.82ppm (1H, dt, J=4.8, 9.6Hz); in 13C-NMR spectrum, a oxycarbon si...
Embodiment 3
[0063] Structural identification of compound 2:
[0064] Compound 2 is a white solid, HR-ESI-MS gives 503.3401 ([M-H]-), combined with 1H-NMR and 13C-NMR to deduce that the molecular formula is C30H48O6. In 1H-NMR (600MHz, pyridine-d5), the compound is shown With 6 methyl hydrogen atom signals δ1.37(6H,s), δ1.14(3H,s), δ1.07(3H,s), δ1.00(3H,d,6.6), δ0.92( 3H,d,6.6); there is an active hydrogen signal δ5.61(o) at the low field, which may be the hydrogen on the double bond. 13C-NMR shows two unsaturated carbon atoms δ126.4 and δ139.5, and one carboxyl carbon atom δ180.5. Based on the above information, it can be inferred that the compound is a ursane-type pentacyclic triterpene acid compound.
[0065] According to HSQC and HMBC spectra, the hydrocarbons of compound 2 can be further assigned.
[0066] Compared with asiatic acid, in 1H-NMR (600MHz, pyridine-d5), compound 2 has a hydrogen signal at 4.45ppm (1H, dd, J=4.2, 10.8Hz); in 13C-NMR spectrum, at 73.4ppm A oxycarbon sig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com