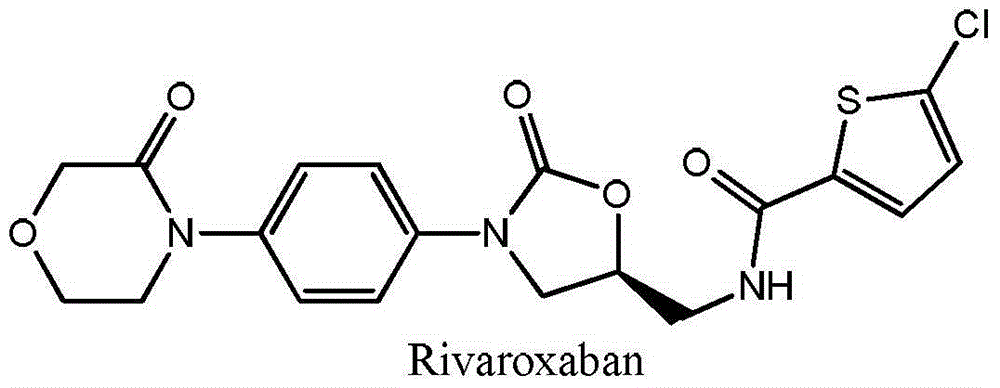
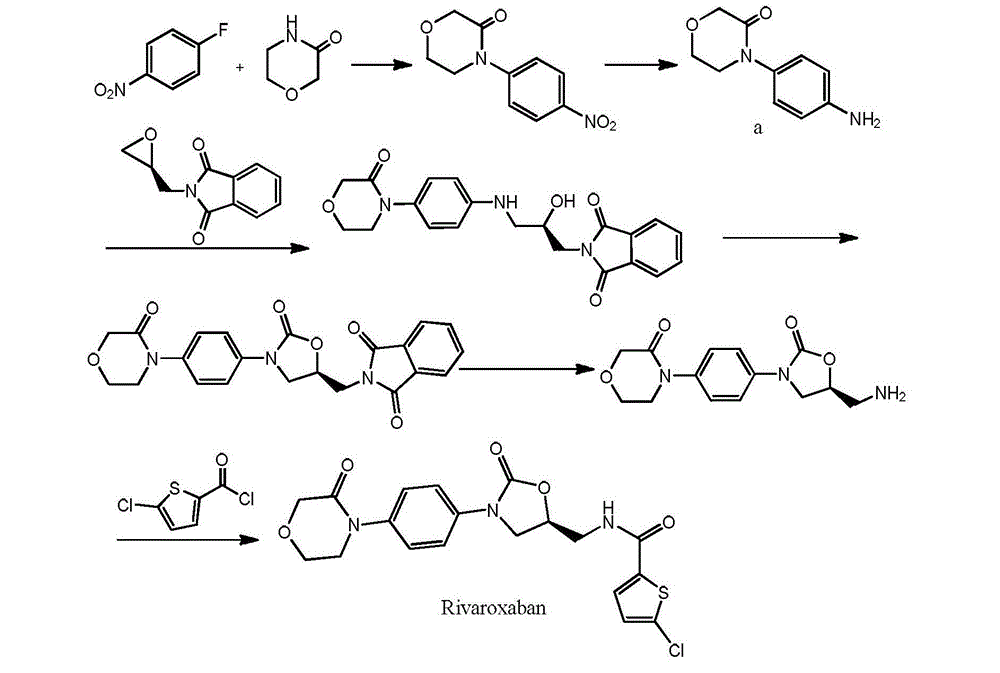
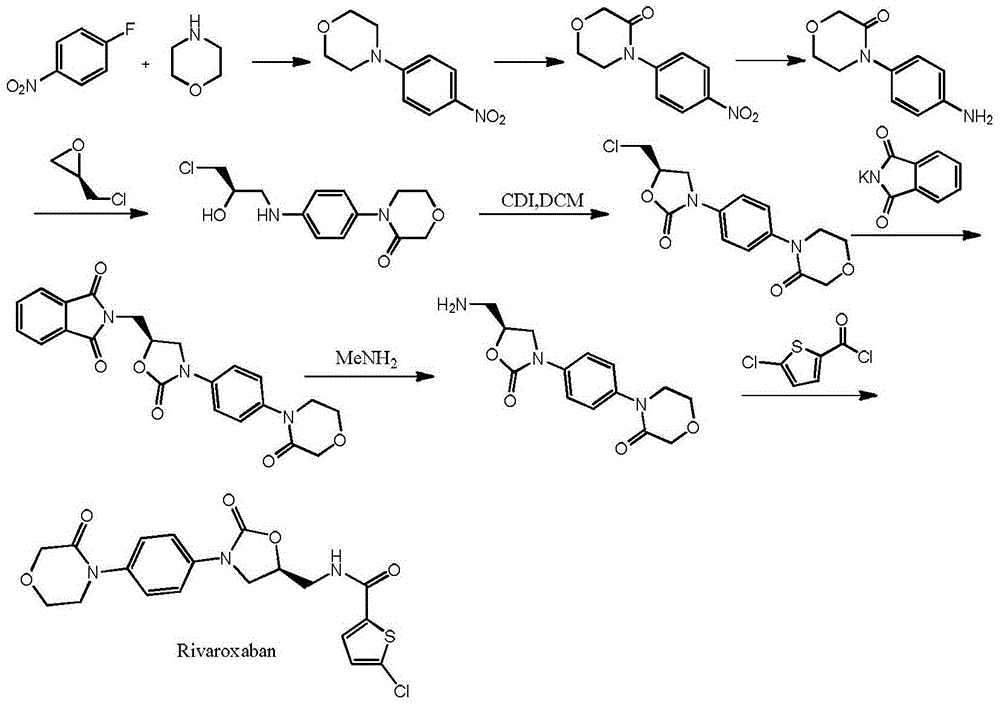
Preparation method of rivaroxaban
A technology for rivaroxaban and intermediates, applied in the field of preparation of rivaroxaban, can solve the problems of increasing raw material cost and production cost, not being suitable for large-scale production, etc., and achieves low pollution, short route and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1: Preparation of N-(4-aminophenyl)-2-(2-chloroethoxy)acetamide (formula III):
[0060]
[0061] In this embodiment, X=Cl in the above reaction formula.
[0062] Add 194.7g (1.8mol) of 1,4-diaminobenzene, 47.4g (0.6mol) of pyridine and 900ml of tetrahydrofuran into the reaction flask, stir evenly, cool down to 10°C to 20°C, and slowly add 2 -(2-Chloroethoxy)acetyl chloride 46.8g (0.3mol), the temperature of the dropping process was controlled at 10°C to 20°C, and the dropping time was controlled at 5 hours. Ethyl acetate:triethylamine=30:20:1, volume ratio) the raw materials basically disappear, stop the reaction, distill THF under reduced pressure (-0.1MPa~-0.09MPa) and recover excess 1,4-diaminobenzene, Add a mixed solvent of 600ml ethyl acetate and 300ml acetone, raise the temperature and reflux to dissolve the residual oil, drop to about 10°C for crystallization for 5 hours, filter, and dry under reduced pressure to obtain 61.8g of off-white intermedi...
Embodiment 2
[0064] Embodiment 2: Preparation of N-(4-aminophenyl)-2-(2-chloroethoxy)acetamide (formula III):
[0065] Add 129.8g (1.2mol) of 1,4-diaminobenzene, 31.6g (0.4mol) of pyridine and 600ml of tetrahydrofuran into the reaction flask, stir evenly, cool down to 10°C to 20°C, and slowly add 2 -(2-Chloroethoxy)acetyl chloride 31.2g (0.2mol), the temperature of the dropping process was controlled at 10°C to 20°C, and the dropping time was controlled at 3 hours. Ethyl acetate:triethylamine=30:20:1, volume ratio) the raw materials basically disappear, stop the reaction, distill THF under reduced pressure (-0.1MPa~-0.09MPa) and recover excess 1,4-diaminobenzene, Add a mixed solvent of 400ml ethyl acetate and 200ml acetone, raise the temperature and reflux to dissolve the residual oil, drop to about 10°C for crystallization for 5 hours, filter, and dry under reduced pressure to obtain 41.8g of off-white intermediate III, with a molar yield of 91.7 %, HPLC purity 97.8%.
[0066] The detec...
Embodiment 3
[0067] Embodiment 3: Preparation of 4-(4-aminophenyl)-3-morpholinone (formula IV):
[0068]
[0069] Add 45.6g (0.2mol) N-(4-aminophenyl)-2-(2-chloroethoxy)acetamide (formula III) prepared in Example 1, 250ml dichloromethane, 82.8g (0.6mol) potassium carbonate, 6.4g (0.02mol) tetrabutylammonium bromide, stir evenly, cool the reaction system to 0°C to 20°C, keep stirring for 5 hours, control in TLC (dichloromethane:methanol=20: 1, volume ratio), the raw materials basically disappeared. Remove insolubles by filtration, wash the organic layer with 80ml of purified water, separate the liquids, evaporate the organic layer to dryness under reduced pressure (-0.08MPa~-0.06MPa), add 150ml of acetone and stir for 3 hours, precipitate crystals, filter, and dry under reduced pressure to obtain 4-(4-aminophenyl)-3-morpholinone 35.2g, molar yield 91.6%, HPLC purity 98.5%.
[0070] The detection data of the title product obtained by mass spectrometry analysis is as follows: HR-MS (ESI)...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap