Stabilized compositions comprising hyaluronic acid
A technology of hyaluronic acid and composition, applied in the field of stable composition containing hyaluronic acid, capable of solving problems such as frequent administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Examples 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 all show that sucrose Good performance as stabilizing aid in hyaluronic acid polymer solutions containing hyaluronic acid polymer solutions and additionally gels based on hyaluronic acid polymers. Therefore, a preferred stabilizing aid is sucrose. These examples show that the addition of sucrose is effective in conferring a stabilization against a decrease in viscosity from 1.2 to at least 11.3 times under the conditions of the examples under which the assay was carried out. Other preferred adjuvants include mannitol, sorbitol, and N-acetylglucosamine (see, e.g., Examples 5, 6, 13); lactose, dextran-40, PEG 4000, carboxymethylcellulose, TRITON TM - x-100 and glycerol (see, eg, Example 16); trehalose and glucose (see, eg, Example 17).
[0060] Large amounts of stabilizing aids are generally not required to achieve significant stabilization. For example, beneficial effects can be...
Embodiment 1A
[0089] A reduction in the viscosity of cross-linked hyaluronic acid-based compositions containing corticosteroids was observed
[0090] During the investigation of dosage forms containing cross-linked hyaluronic acid-based hydrogels suspended in hyaluronic acid solutions, it was observed that in the presence of corticosteroids, triamcinolone acetonide (TA), The viscosity of the hyaluronic acid component decreased more rapidly with time on storage than the same dosage form in the absence of corticosteroids, triamcinolone acetonide.
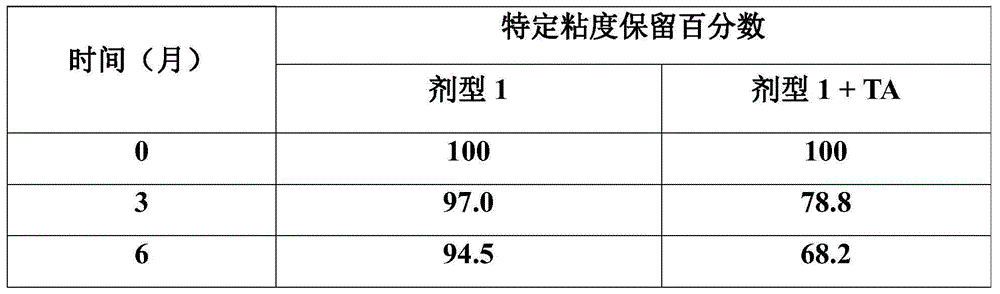
[0091] Table 1
[0092]
[0093] Based on the observations summarized in Table 1, additional studies are needed to determine whether the aforementioned loss of viscosity can be ameliorated, for example, by various means including the introduction of possible stabilizing aids.
Embodiment 1B
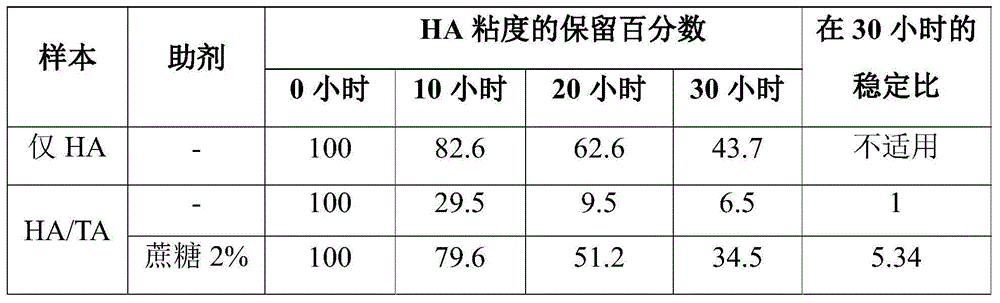
[0095] Effect of sucrose on the viscosity of HA at 80℃
[0096] 6.9 g hyaluronic acid, HA (Lifecore, 888 KDa) was dissolved in 881 mL 0.9% NaCl overnight. The dissolved HA was sterilized by filtration through a 0.2 μm filter (Millipore, Opticap 4”). 3.97 mL of 1 M sodium phosphate with a pH value of 6.71 was added to the HA solution. Then, 372 mg of USP grade triamcinolone acetonide (TA) was mixed with 223.2 mL of dissolved HA was mixed in sterile 250 mL Nalgene bottles. About 1.5 g of sucrose was added to separate samples of 74 ± 1 mL of HA / TA slurry to provide about 2% (w / w) adjuvant Samples. Each mixture was mixed for 1 hour. No auxiliaries were added to the control samples. For each large sample, 2 ± 0.2 mL aliquots were placed in sterile 4 mL glass vials and then closed using septa and aluminum caps It was sealed. All of these aliquots, except the 0 hour sample, were placed in an oven at 80°C. Except for the 0 hour sample, 3 samples were removed from the oven at each tim...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water solubility | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com