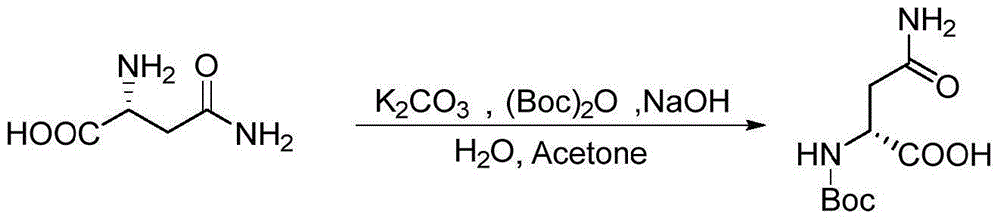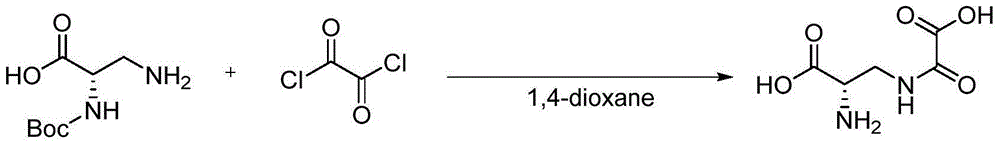Improved synthesis method for dencichine
A synthesis method and technology of Panax notoginseng, applied in the field of medicine, can solve the problems of low extraction rate of Panax notoginseng, small profit margin and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0018] The synthesis method of notoginseng is obtained: take 6.01g of sodium hydroxide, dissolve it in 100mL of water, cool down to 0 degrees, add 20g of L-asparagine, stir for 30 minutes, add 200mL of acetone and 31.4g of K 2 CO 3 , keep the temperature not higher than 5 degrees, add 39.6g di-tert-butyl dicarbonate dropwise, rise to room temperature and react for 24 hours, add 300mL water to dissolve, adjust the pH to 6-7 with 0.5mol / L HCl, extract (dichloro Methane:methanol, 4:1, 200mL×5), retain the aqueous phase, adjust the pH of the aqueous phase to 3-4 with 0.5mol / L HCl, extract (dichloromethane:methanol, 4:1, 200mL×10), no After drying with sodium sulfate and spin-drying, 33g of Boc-L-asparagine was obtained. 33g of Boc-L-asparagine was dissolved in 96mL of ethyl acetate, 96mL of acetonitrile, 1.0mL of saturated citric acid and 48mL of water. After cooling to 0°C, Add 33.3g of diacetoxyiodobenzene in batches, rise to room temperature and react for 4 hours, a solid prec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com