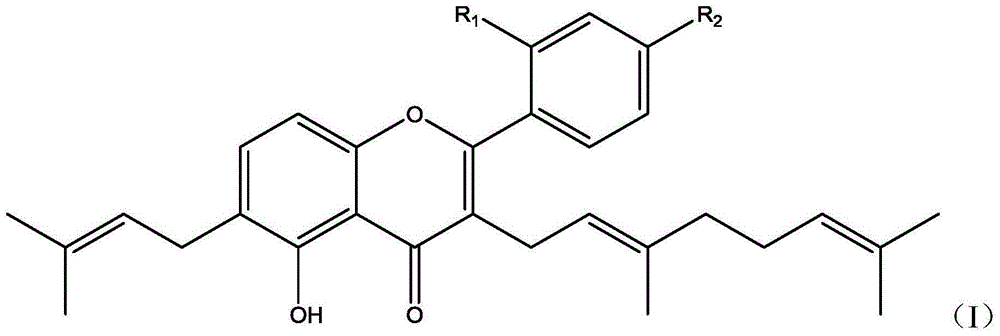
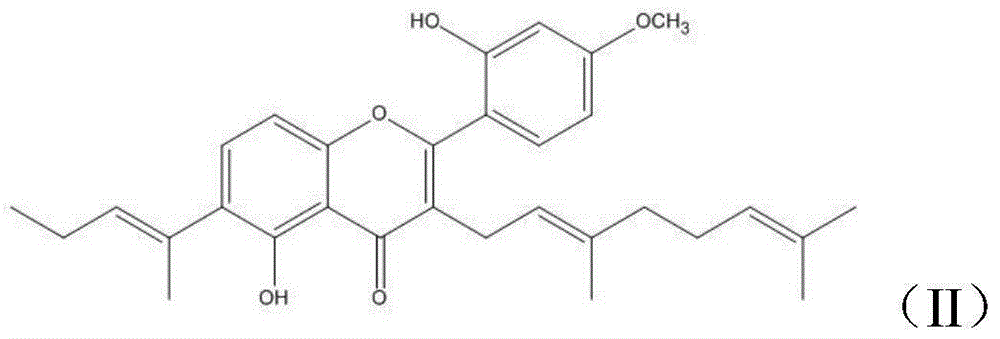
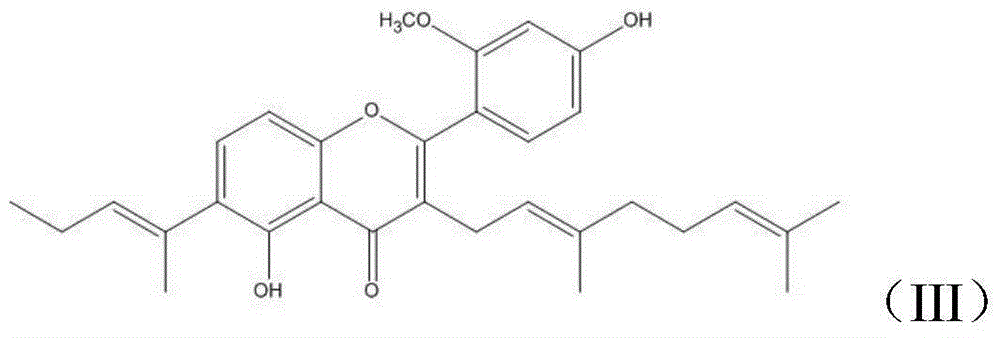
Derivative with 5,2'-dihydroxy-4'-methoxy-3-geranylflavonoids skeleton, and preparation method and applications thereof
A technology of geranyl flavonoids and derivatives, applied in organic chemistry, drug combination, antineoplastic drugs, etc., can solve the mechanism of action, material basis without clear theoretical explanation, structural formula, targeting uncontrollability, flavonoids Compound structure is complex and other issues, to achieve the effects of weak cytotoxicity, good shape, and enhanced biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Derivatives with 5,2'-dihydroxy-4'-methoxyl-3-geranylflavone skeleton, target product II, target product III, the structural formula is as follows:
[0037]
[0038]
[0039] The preparation method is as follows:
[0040] Step 1. Dissolve 2.205g of 2,4-dihydroxyacetophenone in acetone solution, add 2.057g of potassium carbonate and stir to reflux for 8 minutes, add dropwise 2.012g of prenyl bromide to reflux for 8 hours, cool to room temperature, and remove the solvent under reduced pressure. Extract the organic phase with ethyl acetate, wash the organic phase with water and saturated brine successively, dry over anhydrous sodium sulfate, and then carry out column chromatography V (石油醚) :V (乙酸乙酯) =10:1, the first intermediate product (1.113g yield 34.23%) of yellow liquid was obtained, and the structure was confirmed by IR, NMR and MS analysis, and the structural formula was as follows:
[0041]
[0042] Step 2. Dissolve 0.789g of the first intermediate produ...
Embodiment 2
[0057] Derivatives with 5,2'-dihydroxy-4'-methoxyl-3-geranylflavone skeleton, target product II, target product III, the structural formula is as follows:
[0058]
[0059]
[0060] The preparation method is as follows:
[0061] Step 1. Dissolve 1.812g of 2,4-dihydroxyacetophenone in acetone solution, add 1.523g of potassium carbonate and stir to reflux for 12min, add dropwise 3.012g of prenyl bromide to reflux for 12h, cool to room temperature, and remove the solvent under reduced pressure. Extract the organic phase with ethyl acetate, wash the organic phase with water and saturated brine successively, dry over anhydrous sodium sulfate, and then carry out column chromatography V (石油醚) :V (乙酸乙酯) =10:1, the first intermediate product (1.121g yield 35.52%) of yellow liquid was obtained, and the structure was confirmed by IR, NMR and MS analysis, and the structural formula was as follows:
[0062]
[0063] Step 2. Dissolve 0.521g of the first intermediate product in anhy...
Embodiment 3
[0078] Derivatives with 5,2'-dihydroxy-4'-methoxyl-3-geranylflavone skeleton, target product II, target product III, the structural formula is as follows:
[0079]
[0080]
[0081] The preparation method is as follows:
[0082] Step 1. Dissolve 2.000g of 2,4-dihydroxyacetophenone in the acetone solution, add 1.816g of potassium carbonate and stir to reflux for 10min, add dropwise 2.549g of prenyl bromide to reflux for 10h, cool to room temperature, and remove the solvent under reduced pressure. Extract the organic phase with ethyl acetate, wash the organic phase with water and saturated brine successively, dry over anhydrous sodium sulfate, and then carry out column chromatography V (石油醚) :V (乙酸乙酯) =10:1, the first intermediate product (1.146g yield 39.59%) of yellow liquid was obtained, and the structure was confirmed by IR, NMR and MS analysis, and the structural formula was as follows:
[0083]
[0084] Step 2. Dissolve 0.681g of the first intermediate product in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com