Preparation method for DC-CIK cells originated from umbilical cord blood mononuclear cells and preparation
A technology of DC-CIK and nuclear cells, which is applied in the field of immune cell preparation and co-cultivation, can solve the problems of low cell proportion, low cell activity, and poor antigen presentation ability, so as to improve the proliferation ability and tumor killing activity , Improving the production efficiency and the effect of improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0053] Example 2 Mononuclear cells in vitro induced differentiation DC
[0054] 1. Put 4×10 6 pcs / ml~5×10 6 cells / ml cell suspension into 6-well plates (not for tissue culture), 2ml per well, placed at 37°C, 5v / v% CO 2 in the incubator;
[0055] 2. After incubation for 2 hours, gently shake the 6-well plate and aspirate the suspended cells;
[0056] 3. Use X-VIVO TM 15 Serum-free culture medium (containing 0.5v / v~1v / v% umbilical cord blood plasma) washes the 6-well plate 1-2 times, and adds factors GM-CSF (25ng / ml) and IL-4 (16ng / ml) to the adherent cells ml) and Flt3L (50ng / ml) combined differentiation for 24 hours to 48 hours;
[0057] 4. Change the medium every other day, and stabilize the concentration of GM-CSF, IL-4 and Flt3L to generate iDC;
[0058] 5. Then use maturation stimulating factors TNF-α (100ng / ml), IL-1β (10ng / ml), IL-6 (10ng / ml), PGE2 (1μg / ml) for 24 hours to 48 hours to obtain mDC;
[0059] 6. Take the adherent cells just after separation and adhere...
Embodiment 3
[0060] Example 3 Detection of iDC and mDC immunophenotype
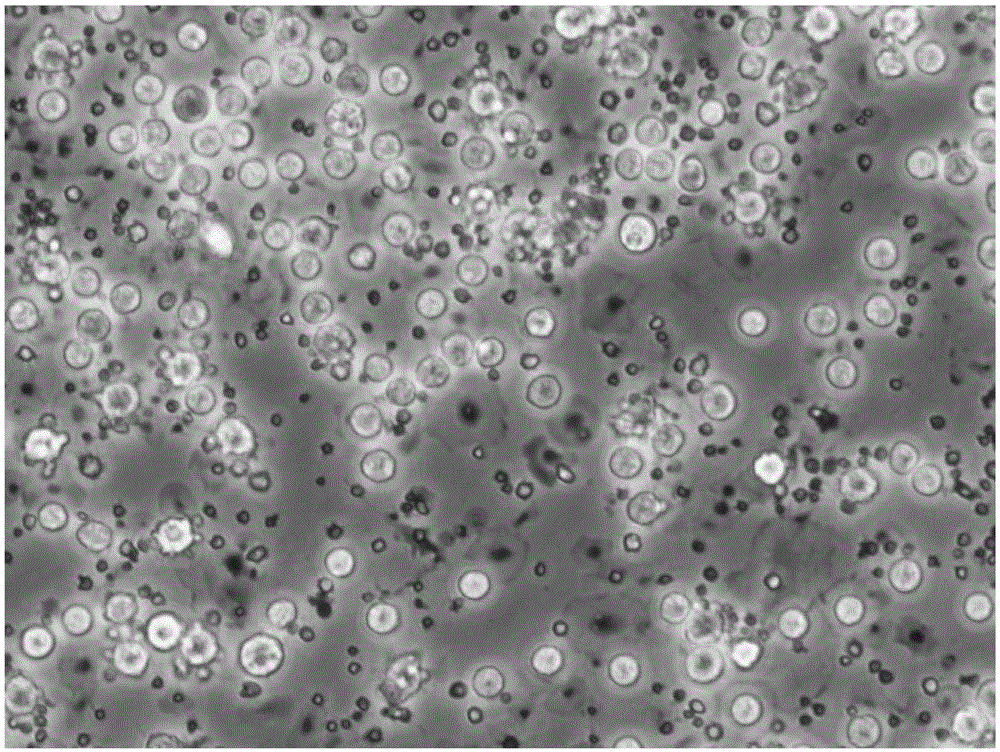
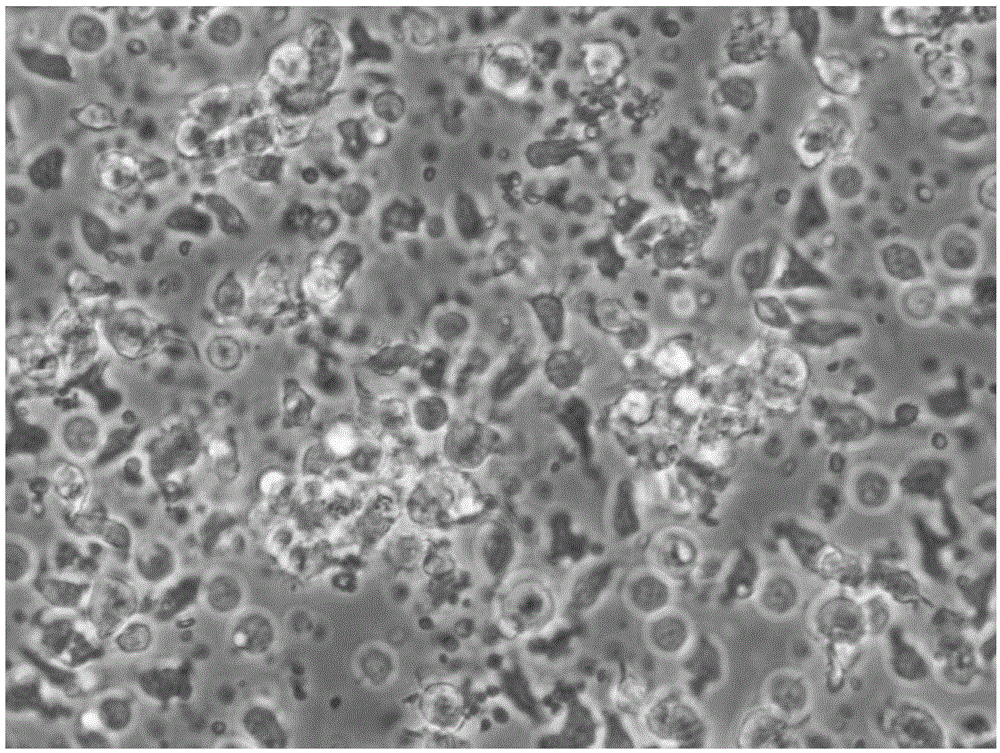
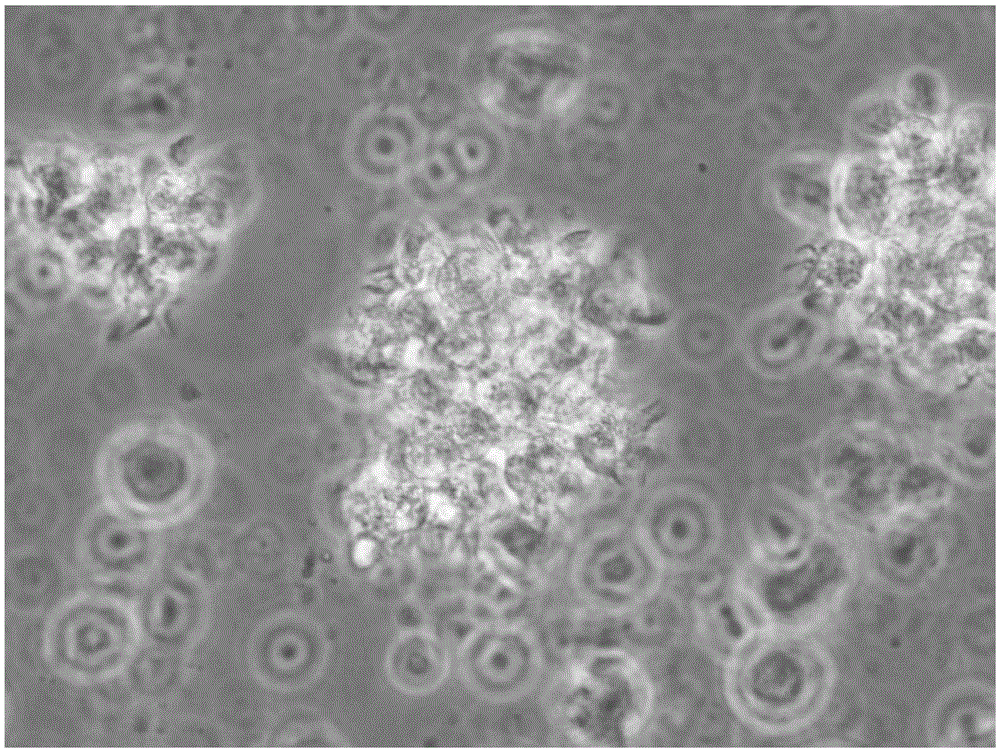
[0061] Take the adherent cells just after 2 hours of separation and adherent cells, and the DC cells at the 48th and 96th hours of culture, wash them twice with PBS+2mM EDTA solution, and take 1×10 cells each. 5 Each / ml were added to the corresponding flow tubes. Add 10 μl of the monoclonal antibodies to be detected, including HLA-DR, CD80, CD83, CD86 and CD14 antibodies, incubate at 4°C in the dark for 10 minutes, wash once with PBS, resuspend in 400 μl of PBS, and use the flow cytometer FACSCalibur (BD company) detection, see the results Figure 3 ~ Figure 6 and table 1
[0062] Table 1 Immunophenotypic determination of DC at different times (%)
[0063]
[0064] Among surface antigens, CD83 is a membrane marker molecule unique to mature DC cells, CD80 and CD86 are co-stimulatory molecules of DC cells, and HLA-DR is an immunostimulatory molecule of DC cells. From the results shown in Table 1, it can be seen t...
Embodiment 4CI
[0065] Embodiment 4 CIK cell preparation
[0066] 1. Take the suspension cells sucked out after incubation for 2 hours in Example 2 for CIK culture;
[0067] 2. Use X-VIVO TM 15 Resuspend in serum-free medium (0.5v / v%~1v / v% umbilical cord blood plasma), the cell concentration is 1×10 6 pcs / ml~3×10 6 cells / ml into culture flasks, add cytokine IFN-γ (25ng / ml), place at 37°C, 5% CO 2 in the incubator;
[0068] 3. Add Anti-CD3mAb (100ng / ml), IL-2 (100ng / ml), IL-1α (5ng / ml) the next day, place at 37°C, 5% CO 2 Continue culturing in the incubator for 4 to 6 days;
[0069] 4. Replenish or subculture every 2 to 3 days, and keep the IL-2 concentration stable.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com