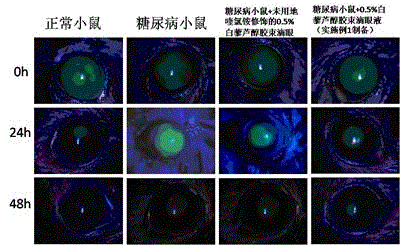
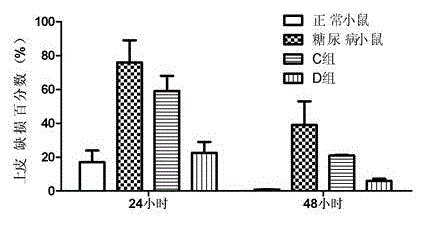
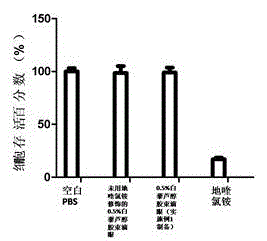
Resveratrol micelle eye drops and preparation method thereof
A technology of resveratrol and eye drops, applied in the directions of emulsion delivery, metabolic diseases, sensory diseases, etc., can solve the problems of only 3 months of effective stability, limited therapeutic effect, no mitochondrial targeting, etc. Prolonged drug action time, good solubility and good drug stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0020] Example 1:
[0021] Put 50mg resveratrol, 25mg dequinium chloride and 1g polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol (molecular weight 118000) in a 100mL round bottom flask, add 10mL absolute ethanol, after fully dissolving, 40 Dehydrated ethanol was evaporated in a rotary vacuum in a water bath to obtain a uniformly dispersed film of the drug and the copolymer, then distilled water was added, the film was fully washed, and the micellar solution was obtained by ultrasonicating in a water bath with a power of 200w for 15 min. After passing the micelle solution through a 0.22 μm microporous membrane, freeze-drying was performed to obtain micelle powder. Then, use 10 mL of borate buffer to disperse and dissolve the micellar powder, add the preservative benzalkonium chloride, and the isotonicity regulator sodium chloride, adjust the pH to 7.4, and sterile filter and pack. The average particle size of resveratrol micelles measured by laser particle size an...
Example Embodiment
[0022] Example 2:
[0023] Put 50mg resveratrol, 26.7mg dequinium chloride and 1.2g polyvinylcaprolactam-polyvinyl acetate-polyethylene glycol (molecular weight 118000) in a 100mL round-bottom flask, add 10mL absolute ethanol, and dissolve it fully. , 40 ℃ water bath rotary vacuum evaporation of absolute ethanol to obtain a uniformly dispersed film of drug and copolymer, then add distilled water, fully wash the film, 200w power water bath ultrasonic for 15min, to obtain a micelle solution. After passing the micelle solution through a 0.22 μm microporous membrane, freeze-drying was performed to obtain micelle powder. Then, use 10 mL of borate buffer to disperse and dissolve the micellar powder, add the preservative benzalkonium chloride, and the isotonicity regulator sodium chloride, adjust the pH to 7.4, and sterile filter and pack. The average particle size of resveratrol micelles measured by laser particle size analyzer was 97 nm, the dispersion index PDI=0.036, and the d...
Example Embodiment
[0024] Example 3:
[0025] Put 50mg resveratrol, 26.2mg dequinium chloride and 1.1g polyvinylcaprolactam-polyvinyl acetate-polyethylene glycol (molecular weight 118000) in a 100mL round-bottom flask, add 10mL absolute ethanol, fully dissolve , 40 ℃ water bath rotary vacuum evaporation of absolute ethanol to obtain a uniformly dispersed film of drug and copolymer, then add distilled water, fully wash the film, 200w power water bath ultrasonic for 15min, to obtain a micelle solution. After passing the micelle solution through a 0.22 μm microporous membrane, freeze-drying was performed to obtain micelle powder. Then, use 10 mL of borate buffer to disperse and dissolve the micellar powder, add the preservative benzalkonium chloride, and the isotonicity regulator sodium chloride, adjust the pH to 7.4, and sterile filter and pack. The average particle size of resveratrol micelles measured by laser particle size analyzer was 94 nm, the dispersion index PDI=0.035, and the dispersio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com