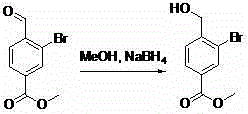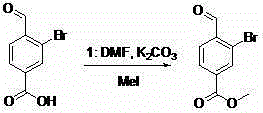Synthesis method for 3-bromine-4-(methylol)methyl benzoate
A technology of methyl aldehyde methyl benzoate and methyl benzoate, which is applied in the field of synthesis of methyl 3-bromo-4-benzoate, can solve the problems of high toxicity, long reaction time, difficult purification and the like, and avoids light exposure. The reaction and synthesis process are safe and reliable, and the effect of simplifying the reaction process and post-processing process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
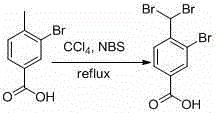
[0026] Add 250g of 3-bromo-4-methylbenzoic acid and 475g of NBS into a 3L three-necked flask, add 2.5L of carbon tetrachloride and 11g of benzoyl peroxide, heat up to 80 degrees and reflux for 2 days, cool to room temperature, filter, and use for cake filtering 2L of carbon tetrachloride was washed three times, and the combined filtrate was spin-dried to obtain about 220 g of crude 3-bromo-4-(dibromomethyl)benzoic acid, which was directly put into the next reaction.
[0027] Add 220g of the above-mentioned prepared 3-bromo-4-(dibromomethyl)benzoic acid crude product in a 3L three-necked flask, add 1.5L ethanol to dissolve, heat up 50 degrees, add dropwise 300ml aqueous solution of 200g silver nitrate, and keep warm for 55 React for 1 hour, cool to room temperature, filter, wash the filter cake three times with 1L ethanol, combine the filtrate and spin dry to obtain about 130g of crude product, the crude product is beaten with 500mL petroleum ether, and filter to obtain about 11...
Embodiment 2
[0031] Add 250g of 3-bromo-4-methylbenzoic acid and 475g of NBS into a 3L three-necked flask, add 2.5L of carbon tetrachloride and 11g of AIBN, heat up to 80 degrees for reflux reaction for 2 days, cool to room temperature, filter, filter the cake with 2L of carbon tetrachloride After washing three times, the combined filtrate was spin-dried to obtain about 210 g of crude product 3-bromo-4-(dibromomethyl)benzoic acid, which was directly put into the next step reaction.
[0032] Add 210 g of the 3-bromo-4-(dibromomethyl) benzoic acid crude product prepared above in a 3 L three-necked flask, add 1.4 L ethanol to dissolve, heat up 50 degrees, add dropwise 300 ml aqueous solution of 200 g silver nitrate, and keep warm for 55 React for 1 hour, cool to room temperature, filter, wash the filter cake three times with 1L ethanol, combine the filtrate and spin dry to obtain about 125g of crude product, use 500mL petroleum ether to beat the crude product, filter to obtain about 110g of 3-...
Embodiment 3
[0036] Add 250g of 3-bromo-4-methylbenzoic acid and 475g of NBS to a 3L three-necked flask, add 2.5L of carbon tetrachloride and 1.1g of AIBN, heat up to 80 degrees and reflux for 2 days, cool to room temperature, filter, filter the cake with 2L of tetrachloride The carbon was washed three times, and the combined filtrate was spin-dried to obtain about 200 g of crude product 3-bromo-4-(dibromomethyl)benzoic acid, which was directly put into the next step reaction.
[0037]Add 200 g of the 3-bromo-4-(dibromomethyl) benzoic acid crude product prepared above in a 3 L three-necked flask, add 1.3 L ethanol to dissolve, heat up 50 degrees, add dropwise 300 ml aqueous solution of 190 g silver nitrate, and keep warm for 55 reaction for 1 hour, cooled to room temperature, filtered, and the filter cake was washed three times with 1L ethanol, and the combined filtrate was spin-dried to obtain 125g of crude product, which was beaten with 500mL petroleum ether, and filtered to obtain about ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com