Method for efficiently preparing linear polarization tree dendritic cells and application of method
A technology of dendritic cells and cells, applied in the biological field, can solve problems such as quality control workload, a large number of cytokines, and titer differences, and achieve the effects of facilitating quality control, simplifying uncertainty, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of T cell culture supernatant
[0038] Peripheral blood mononuclear cells were isolated according to the method described by Jonuleit et al. (GeneTher. 200310(3)). PBMCs were first isolated by Ficoll-Hypaque density gradient centrifugation.
[0039] T cell supernatant culture process:
[0040] a) Isolation of mononuclear cells
[0041] i. Blood transfer: first transfer the peripheral blood to 50ml centrifuge tubes, 20-30ml per tube, and take blood samples for blood components. Mark, trim, centrifuge at 1300g for 10min.
[0042] ii. Prepare lymphocyte separation medium: add 15ml lymphocyte separation medium to each 50ml centrifuge tube.
[0043] iii. Add separation solution: After centrifugation, transfer the upper layer of plasma to a centrifuge tube, keep an appropriate amount for pathogen detection, seal the rest of the plasma with a parafilm, and bathe in water at 56°C for 30 minutes. After transferring the plasma, dilute blood cells with 0.9% sodium...
Embodiment 2
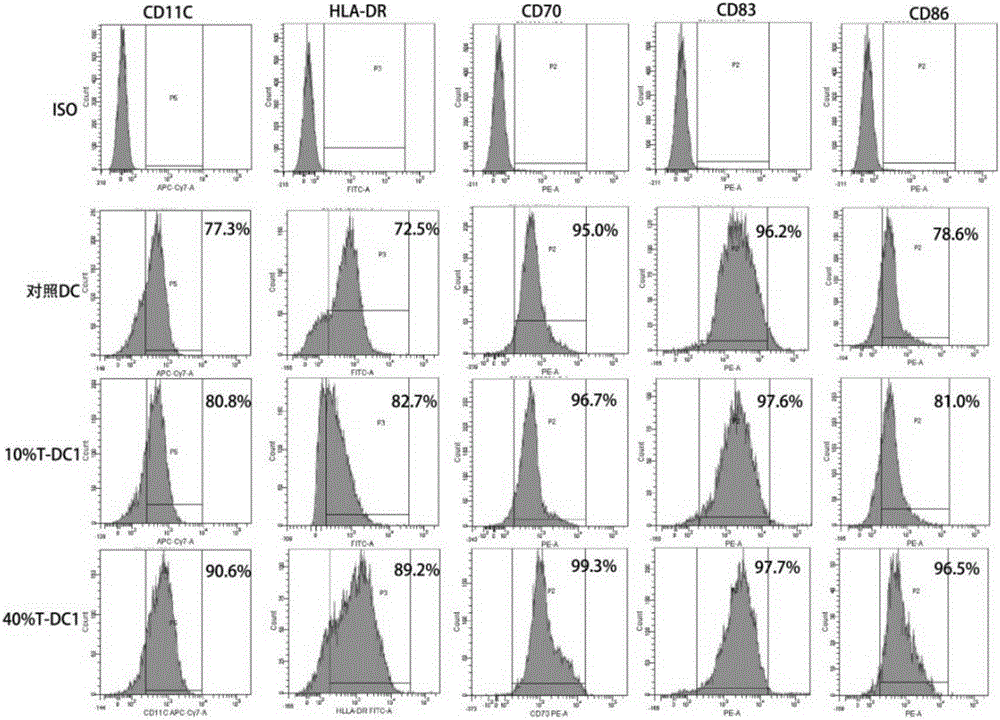
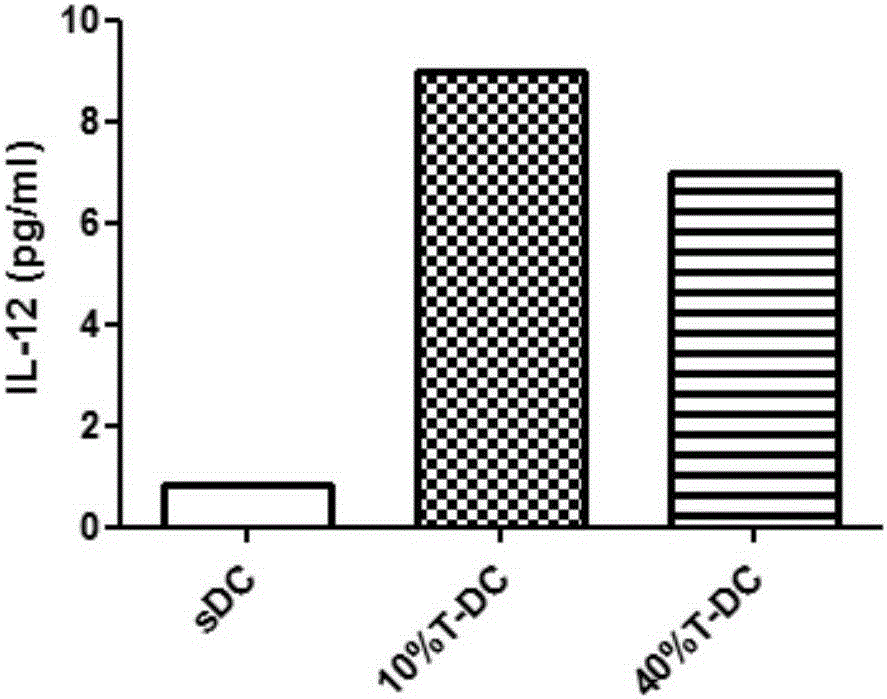
[0063] Preparation of type 1 polarized dendritic cells (type 1 polarized dendritic cell, DC1) derived from adult peripheral blood:
[0064] Peripheral blood mononuclear cells were isolated according to the method described by Jonuleit et al. (GeneTher. 200310(3)). First, peripheral blood mononuclear cells were separated by Ficoll-Hypaque density gradient centrifugation, and plasma was collected for subsequent culture.
[0065] By density 3.0×10 6 / ml, inoculate cells into T75 culture flasks, add 10% plasma to TakaraGT551-H3 medium, place at 37.0°C, saturated humidity, 5% CO 2 cultivated in the environment. After culturing for 3 hours, the suspended lymphocytes were washed away, and a medium containing GM-CSF, IL-4 and 10% plasma was added. Adherent cells were cultured on the 3rd day, and half of the medium was replaced on the 5th day. After changing the medium in half, add 10% T cell supernatant. The cells collected until the sixth day of culture were DC1 cells. After DC...
Embodiment 3
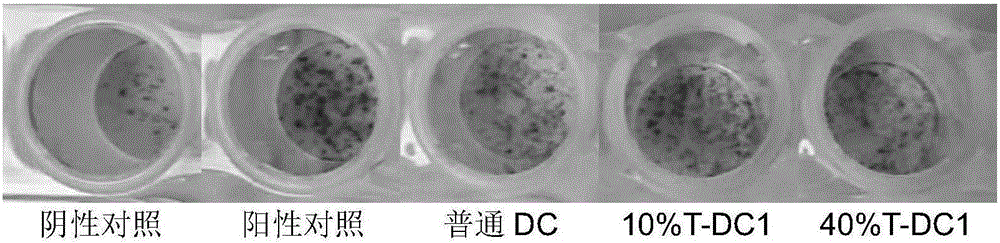
[0067] Tumor antigen-specific CTL cells induced by DC1 cells in vitro
[0068] Peripheral blood mononuclear cells were isolated as described above, cultured briefly in an adherent culture dish, and cells capable of adherence were isolated. Suspended cells at a density of 3.0×10 6 1000U / ml of IL-2 was added to the culture medium. Doubly add medium every two days. On the fifth day of culture, the factor IFN-γ was added, and on the sixth day, the factors IL1-α and CD3 were added.
[0069] The antigen peptide-loaded DC1 obtained in Example 2 was mixed with T cells for culture. Doubly add medium every two days. On the fourth day after the start of the mixed culture, the tumor-specific antigen peptide was loaded again. After the tumor antigen peptide powder in the CTL-TP kit was centrifuged (300g, 10min), 1ml of medium was added to each tube, fully dissolved and mixed, and then added to the culture system. Afterwards, continue culturing until reinfusion, and the T cell culture...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com