In-vitro simulated ventricular system device
A ventricular system, in vitro simulation technology, applied in the field of lateral ventricle external drainage technology and intracranial pressure detection, can solve problems such as adjusting the drainage volume, unable to observe changes in the intracranial ventricular system, etc., to achieve reliable use, avoid direct contact, and reduce risks. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0021] In order to further explain the technical means and effects of the present invention to achieve the intended purpose of the invention, the specific implementation, structure, characteristics and features of the in vitro simulated ventricular system device proposed according to the present invention will be described below in conjunction with the accompanying drawings and preferred embodiments. Efficacy, detailed as follows.
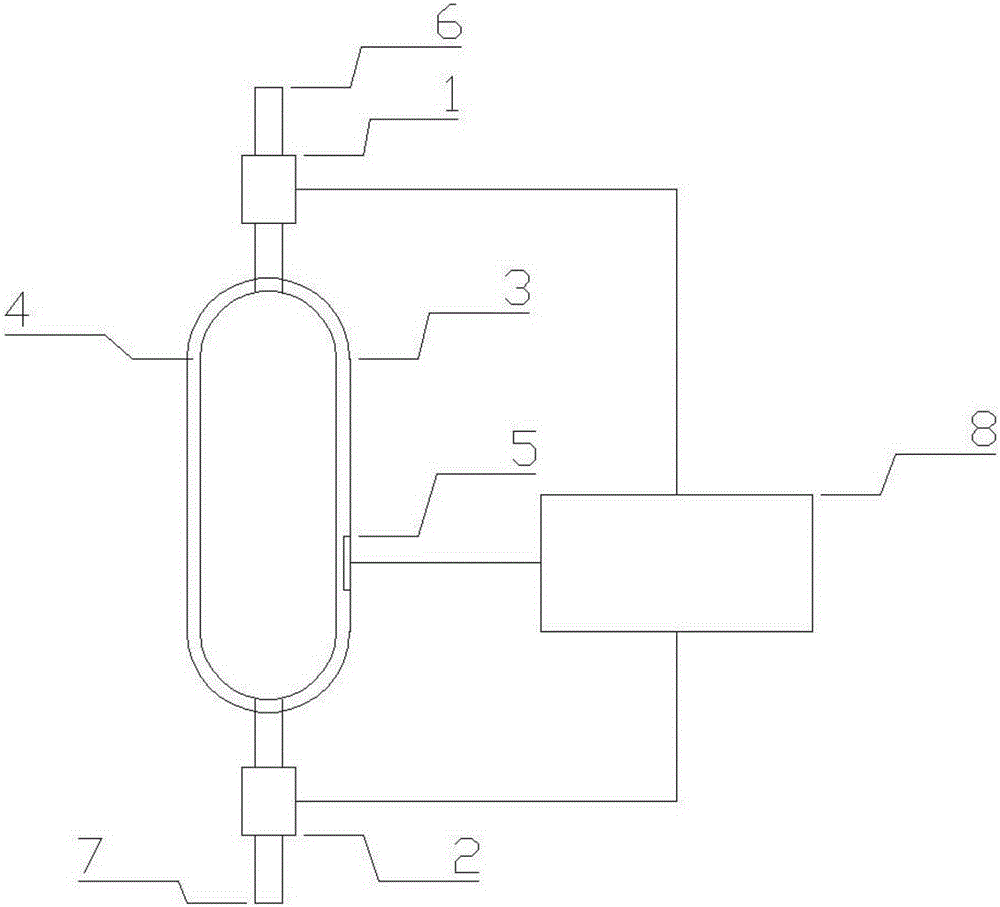
[0022] see figure 1 , a device for simulating a cerebroventricular system in vitro in the present invention includes an outer bag 3, an inner water bag 4, a first pipeline 6, a second pipeline 7 and an outer controller 8, the inner water bag 4 is arranged in the outer bag 3, and the outer bag 3. A pressure sensor 5 is installed on the inner wall; the first pipe 6 communicates with the inner water bag 4 through the outer bag 3 from above, and the upper valve 1 is installed on the first pipe 6; the second pipe 7 passes through from below The outer b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com