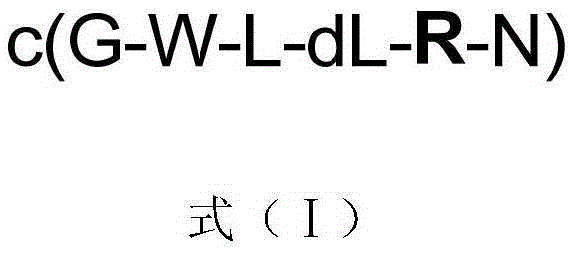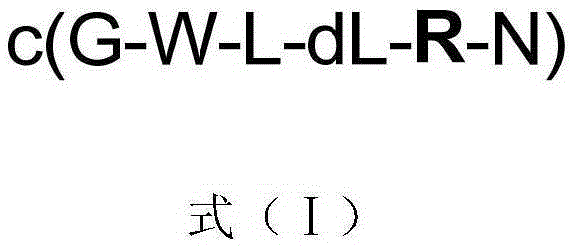Cyclic hexadepsipeptide compound and application thereof in preparing benign prostatic hyperplasia resistant drug
A prostatic hyperplasia, compound technology, application in the preparation of anti-benign prostatic hyperplasia drugs, cyclic hexapeptide compounds and their use in the field of preparation of α1-AR antagonists, can solve problems affecting the quality of life of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Solid phase synthesis and structure identification of compounds 1-5 represented by formula (I)
[0019]
[0020] In formula (I), compound 1: R=Ser / S; or compound 2: R=Asp / D; or compound 3: R=Glu / E; or compound 4: R=His / H; or compound 5: R =Met / M.
[0021] One, the solid-phase synthesis of compound 1-5 as shown in formula (I)
[0022] 1. Solid phase synthesis
[0023] Using solid-phase peptide synthesis technology, N-α-Fmoc-protected amino acid is used as raw material, Fmoc-AA-2CL resin is used as carrier, and HBTU method is used for coupling. The Fmoc-AA-2CL resin (1mmol) was removed with piperidine-DMF (V:V=1:5), and the Fmoc protecting group was removed for 15 minutes. After washing with DCM and DMF, Fmoc protected amino acids (3mmol), HBTU ( 3mmol), DIEA (3mmol) for coupling, 30 minutes, after washing, the steps of removing the Fmoc protecting group-washing-coupling-rewashing are cycled until the last amino acid coupling is completed, and the synthesis of the e...
Embodiment 2
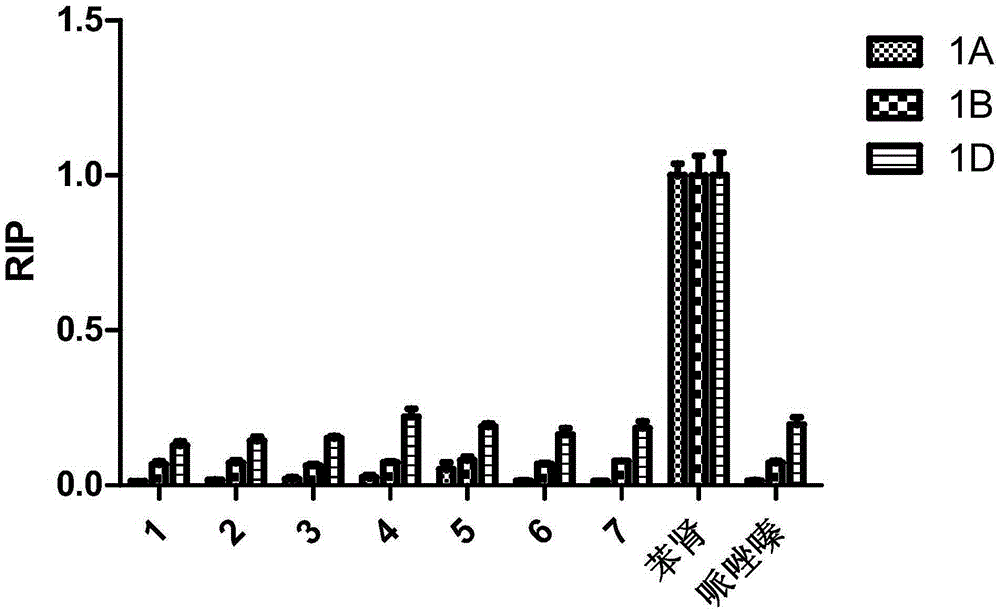
[0044] The cyclic hexapeptide compound-compound 1-5 of embodiment 1 and the same series natural cyclic hexapeptide compound 6 (c(G-W-L-dL-L-allo-Ile-N)) and compound 7 (c(G-W-L-dL-V-N) )) to α 1 -Three subtypes of AR α 1A -, α 1B - and α 1D -AR was tested for selective antagonist activity.
[0045] 1. Compound pair alpha 1 -Investigation on the Selective Inhibition of AR Subtypes
[0046] Strain cultivation and plasmid extraction: The corresponding plasmid (pGL4.29[luc2P / CRE / Hygro] was purchased from PromegaProductIDE8471, which contained the reporter gene luc2p-CRE; pGL4.74[hRluc / TK] was purchased from PromegaProductIDE6921, which contained the reporter gene hRluc -TK; EX-A0967-M29 was purchased from GeneCopoeia ProductIDA0967 containing α 1 The coding gene of A-AR; EX-Y3321-M29 was purchased from GeneCopoeiaProductIDY3321, which contains α 1 The coding gene of B-AR; EX-Y2008-M29 was purchased from GeneCopoeiaProductIDY2008, which contains α 1 D-AR coding gene) strain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com