Highly galactosylated anti-her2 antibodies and uses thereof
A technology of galactosylation and fucosylation, which is used in antitumor drugs, digestive systems, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0208] Example 1: Trastuzumab Transgenicly Produced
[0209] The glycosylation pattern of trastuzumab antibodies produced in the milk of transgenic goats was determined by releasing N-glycans from the antibodies and running the released oligosaccharides on a column ("oligosaccharide tag").
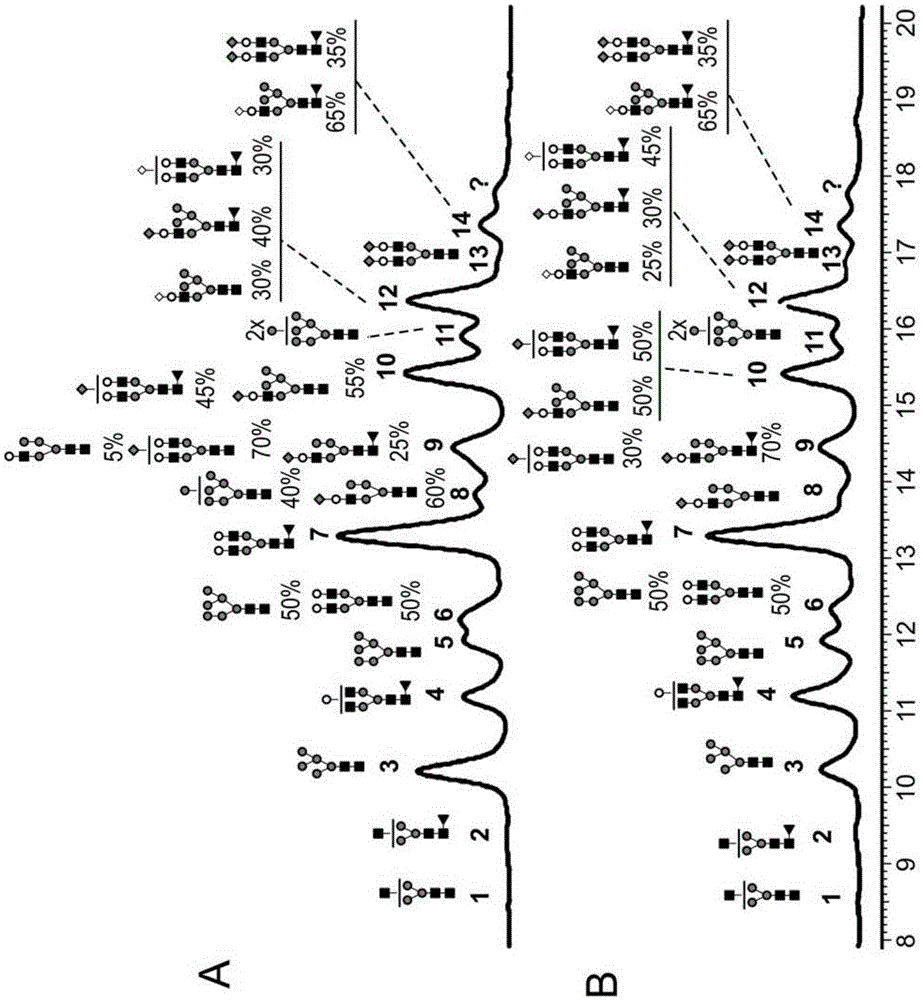
[0210] Figure 1-4 and 6 show that from goat #1 ( Figure 2-4 ) and goat #2 ( figure 1 and 6 ) N-glycan oligosaccharides released from trastuzumab antibodies produced transgenically. Monosaccharide groups are described as follows:
[0211] Black squares: N-acetylglucosamine (GlcNac)
[0212] Triangle: fucose
[0213] Gray circle: Mannose
[0214] White circle: Galactose
[0215] Gray diamonds: N-glycolylneuramic acid (NGNA): a type of sialic acid
[0216] White diamonds: N-acetylneuraminic acid (NANA): a type of sialic acid
[0217] figure 1 Representative chromatograms of N-glycan oligosaccharides released from transgenic trastuzumab antibodies produced in the milk of goat #2 a...
Embodiment 2
[0228] Example 2: Glycosylation Analysis of Trastuzumab Transgenically Produced in Other Animals
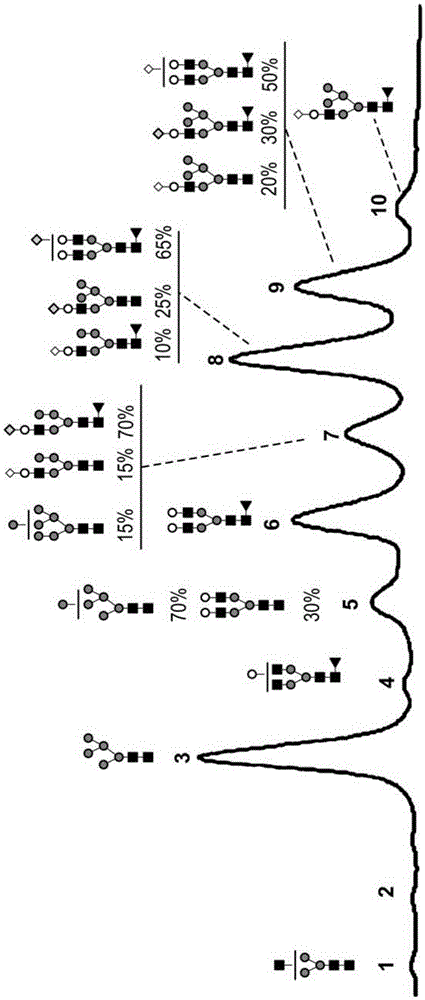
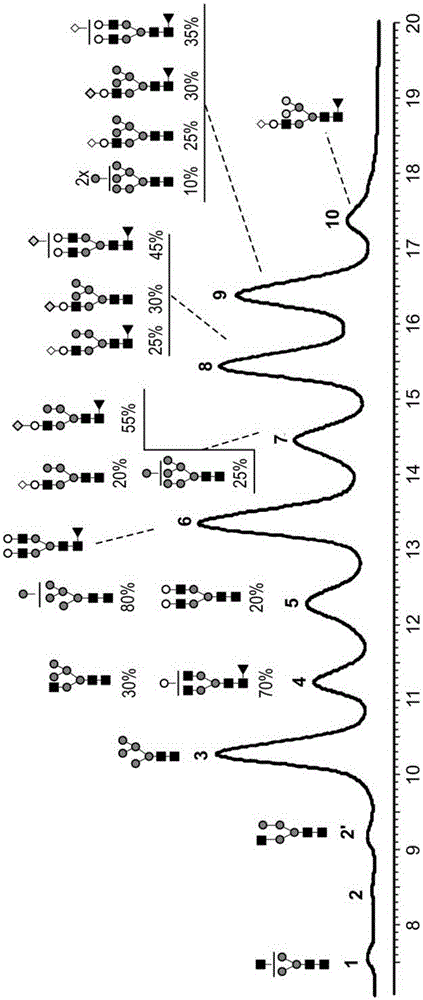
[0229] Figure 9 The relative percentages of the different N-glycan oligosaccharides present in transgenicly produced trastuzumab from the milk of goat #3 on lactation day 7 and goat #4 on day 3 / 4 of lactation are described and also summarized in In Table 3 below:
[0230] Table 3: Summary of Trastuzumab-generated data in goats #3 and #4
[0231]
[0232] *Calculated according to the formula in the manual
[0233] Figure 10 The relative percentages of different N-glycan oligosaccharides present in transgenicly produced trastuzumab antibodies from milk of goat #5 on day 3 of lactation and goat #6 on days 5, 6 and 7 of lactation are described in , and are also summarized in Table 4 below:
[0234] Table 4: Summary of data for Trastuzumab produced in goats #5 and #6
[0235]
[0236] *Calculated according to the formula in the manual
[0237] Figure 11 The relative p...
Embodiment 3
[0247] Characterization of the trastuzumab produced by the transgene of embodiment 3
[0248] Functional characterization of transgenically produced trastuzumab produced in goat milk with commercial / trastuzumab comparison. Binding affinities to HER2-expressing cell lines, CD16 on NK cells, and C1q were quantified. In addition, these antibodies were evaluated for their ability to induce lysis of HER2-expressing cell lines by antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC), as well as their ability to inhibit cell proliferation.
[0249] For transgenic produced trastuzumab lot A, transgenic produced trastuzumab lot B and commercial / trastuzumab (Roche), antigen recognition on the HER2-expressing SK-BR-3 cell line was of the same order (arbitrary dissociation constant, Kd, 2-6 μg / ml). Transgenicly produced trastuzumab antibody with an IC of 30 μg / ml (for batch A) and 25 μg / ml (for batch B) 50 Values bind to the CD16 rece...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com