Application of pharmaceutical composition to prepare medicines treating cutaneous vasculitis
A kind of technology of skin vasculitis and composition, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Comparison of acute toxic effects of oxymatrine, glycyrrhizic acid and their compositions on mice
[0024] Kunming mice were randomly divided into a normal control group and a test drug group, with ten mice in each group, half male and half male. In addition to the normal control group, the test drug group was injected intraperitoneally (ip) with a large dose of oxymatrine, glycyrrhizin and the combination of the two components once, and the animals were observed continuously for 7 days, and the death time and number of animals were recorded.
[0025] The results showed that when the dose of oxymatrine was 950mg / kg, it had great toxicity, and 9 of the 10 mice died; when the dose of glycyrrhizin was 950mg / kg, 2 of the 10 mice died, while the oxidized Kushen minus: glycyrrhizin ratio 1:1 and combined dose of 950mg / kg and matrine: glycyrrhizin ratio of 2:1 and combined dosage of 950mg / kg, no animals died, oxidized matrine reduced: glycyrrhizin When the ratio of oxymatri...
Embodiment 2
[0030] Clinical observation of compound glycyrrhizin combined with oxymatrine in the treatment of allergic cutaneous vasculitis
[0031] 1 Materials and methods
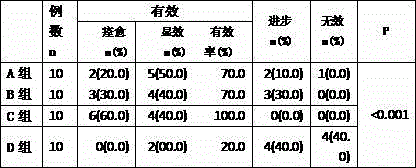
[0032] 1.1 Clinical data The clinical diagnosis conforms to 40 patients with allergic cutaneous vasculitis, 20 males and 20 females, aged 20 to 50 years old, who have not received treatment within one month, and have no serious visceral diseases such as liver and kidney damage, and no Active pulmonary tuberculosis, hypertension, diabetes, cataract history, excluding pregnant and lactating women. The patients were randomly divided into four groups: oxymatrine treatment group (group A), compound glycyrrhizin treatment group (group B), oxymatrine combined with compound glycyrrhizin treatment group (group C), and control group (group D). group), ten cases in each group, half male and half male. There was no statistical difference among the four groups in terms of age, gender, and disease severity (P>0.05).
[0033] ...
Embodiment 3
[0044] Observation on the curative effect of compound glycyrrhizin combined with oxymatrine in the treatment of pigmented purpuric dermatitis
[0045] 1 Materials and methods
[0046] 1.1 Clinical data Clinical diagnosis of 10 patients with pigmented purpura-like dermatitis, 6 males and 4 females, aged 24 to 60 years old, never received systemic treatment within one month, no photosensitivity disease, no liver and kidney damage, etc. Severe visceral disease, no active tuberculosis, cataract history.
[0047] 1.2 Treatment method The patients were given intravenous infusion of oxymatrine injection 0.6g100ml / d and compound glycyrrhizin 100mg / day, both taking 6 weeks as a course of treatment, and the curative effect was judged after the course of treatment was over.
[0048] 1.3 Criteria for judging the curative effect: clinical recovery means that the skin lesions have disappeared after treatment by more than 90%; markedly effective means that the skin lesions have disappeare...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com