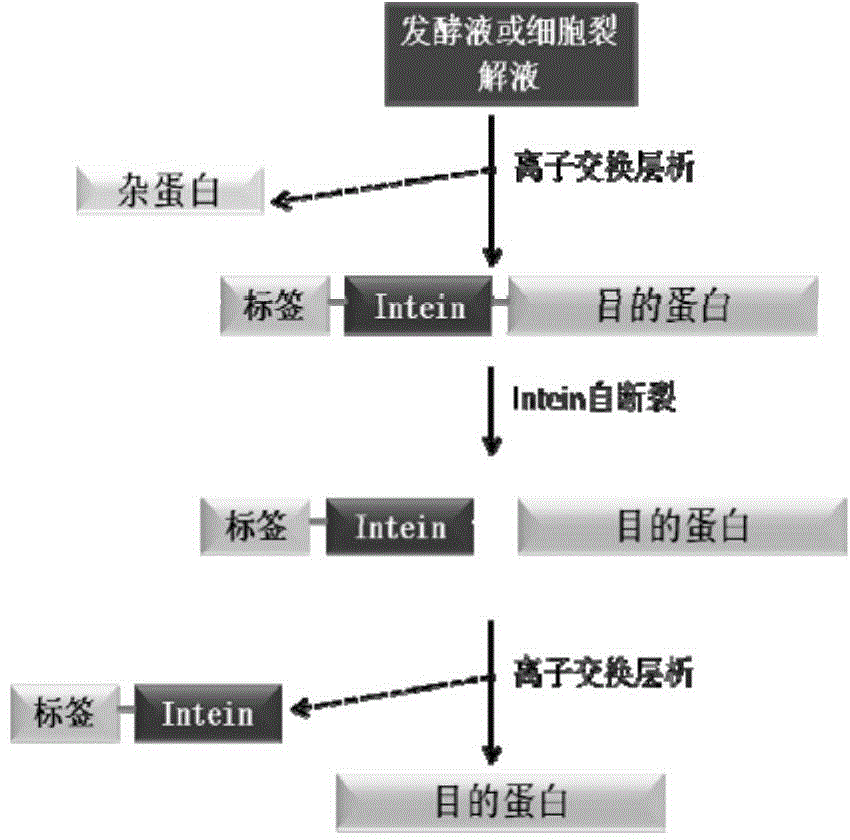
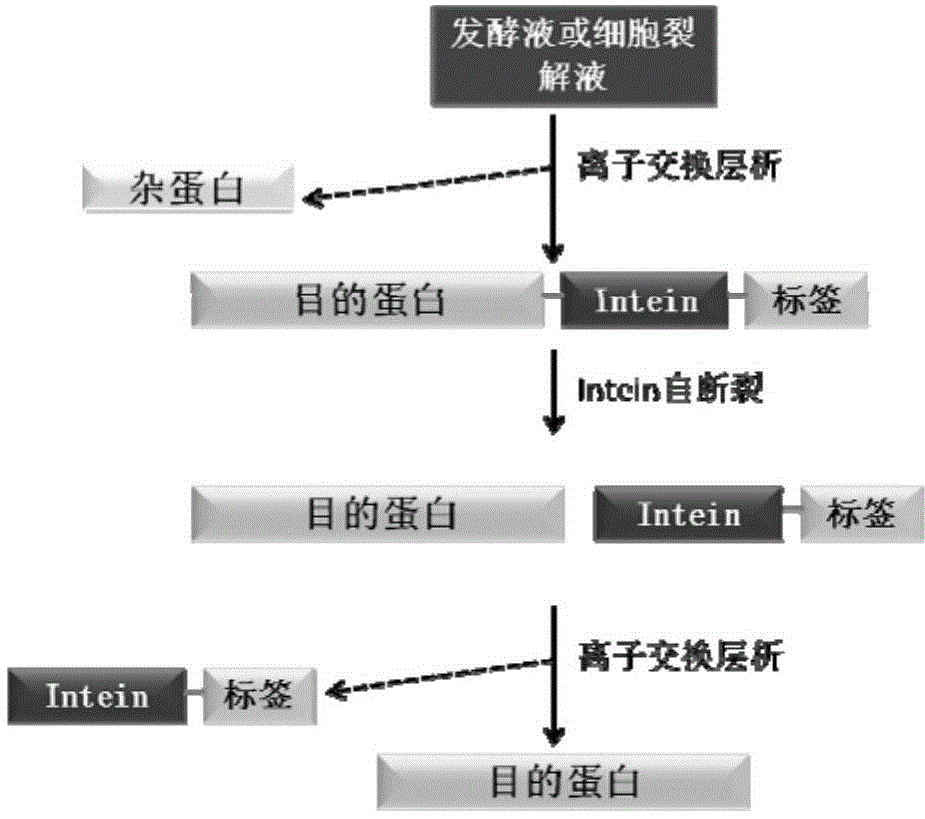
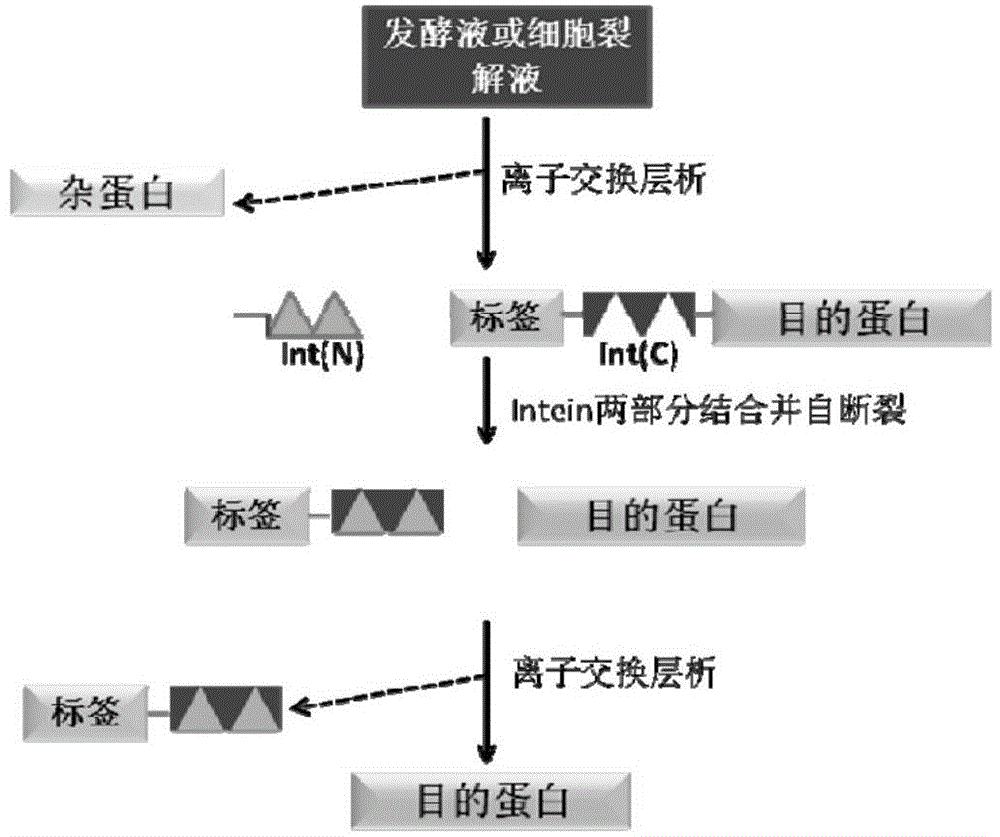
Fusion expression and purification method for recombinant proteins by aid of alkaline tags and intein
A technology for expressing, purifying and fusion proteins, which can be used in chemical instruments and methods, recombinant DNA technology, animal/human proteins, etc., and can solve the problems of difficult industrial production, low enzyme digestion efficiency, and high cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1H
[0062] Example 1 His fusion with ΔI-CM to express Interleukin
[0063] The His gene sequence used in this experiment is as shown in SEQ ID NO.5, specifically:
[0064] CACCATCATCATCATCAT. According to the information of JBacteriol (1991) 173 (18): 5653-5662 and BiotechnolProg, 2000, 16: 1055-1063, the transformed form ΔI-CM of MtuRecAintein is obtained, and the coding gene sequence of the ΔI-CM is shown in SEQ ID NO.2 ). ΔI-CM is one of the most studied inteins at present. Its activity is the highest at pH 6.0-6.5, and it has C-terminal cleavage activity. Therefore, the target protein Interleukin (interleukin) fused and expressed at the C-terminus can be released separately to obtain Zbasic- Intein and Interleukin two parts. The coding gene sequence of Interleukin (interleukin-15) is shown in SEQ ID NO.3.
[0065] The genes of His, ΔI-CM and Interleukin were cloned and connected together by bridge PCR, and the obtained His-ΔI-CM-Interleukin was sequenced from the N-termina...
Embodiment 2Zba
[0070] Example 2 Zbasic and ΔI-CM fusion tag express Interleukin
[0071] (1) Construction of Zbasic and ΔI-CM fusion tag expression vector and host bacteria
[0072] According to the information reported in the document JChromatogrA (2002) 942 (1-2): 157-66, the DNA sequence of Zbasic was synthesized (Nanjing GenScript Co., Ltd.), and the coding gene sequence of Zbasic is shown in SEQ ID NO.1.
[0073] According to the information of JBacteriol (1991) 173 (18): 5653-5662 and BiotechnolProg, 2000, 16: 1055-1063, the transformed form ΔI-CM of MtuRecAintein is obtained, and the coding gene sequence of the ΔI-CM is shown in SEQ ID NO.2 . ΔI-CM is one of the most studied inteins at present. Its activity is the highest at pH 6.0-6.5, and it has C-terminal cleavage activity. Therefore, the target protein Interleukin (interleukin) fused and expressed at the C-terminus can be released separately to obtain Zbasic- Intein and Interleukin two parts. The coding gene sequence of Interle...
Embodiment 3
[0084] Example 3 MBP and ΔI-CM fusion tag expresses Interleukin
[0085] (1) Construction of MBP and ΔI-CM fusion tag expression vector and host bacteria
[0086] The gene expression sequence of MBP is cloned from the carrier pMAL-p2X of NEB Company (NewEngland Biolabs), and the coding gene sequence of the MBP is shown in SEQ ID NO.7, specifically:
[0087] ATGAAAATAAAAACAGGTGCACGCATCCTCGCATTATCCGCATTAACGACGATGATGTTTTCCGCCTCGGCTCTCGCCAAAATCGAAGAAGGTAAACTGGTAATCTGGATTAACGGCGATAAAGGCTATAACGGTCTCGCTGAAGTCGGTAAGAAATTCGAGAAAGATACCGGAATTAAAGTCACCGTTGAGCATCCGGATAAACTGGAAGAGAAATTCCCACAGGTTGCGGCAACTGGCGATGGCCCTGACATTATCTTCTGGGCACACGACCGCTTTGGTGGCTACGCTCAATCTGGCCTGTTGGCTGAAATCACCCCGGACAAAGCGTTCCAGGACAAGCTGTATCCGTTTACCTGGGATGCCGTACGTTACAACGGCAAGCTGATTGCTTACCCGATCGCTGTTGAAGCGTTATCGCTGATTTATAACAAAGATCTGCTGCCGAACCCGCCAAAAACCTGGGAAGAGATCCCGGCGCTGGATAAAGAACTGAAAGCGAAAGGTAAGAGCGCGCTGATGTTCAACCTGCAAGAACCGTACTTCACCTGGCCGCTGATTGCTGCTGACGGGGGTTATGCGTTCAAGTATGAAAACGGCAAGTACGACATTAAAGACGTGGGCGTGGATAA...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com