Construction method of hepatitis vaccine sensitization dendritic cell inducing specific T cell
A dendritic cell and specific technology, applied in the field of medicine and biology, can solve the problems of difficult virus removal, low clinical cure rate, high recurrence rate, etc., and achieve the effect of improving specific cellular immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
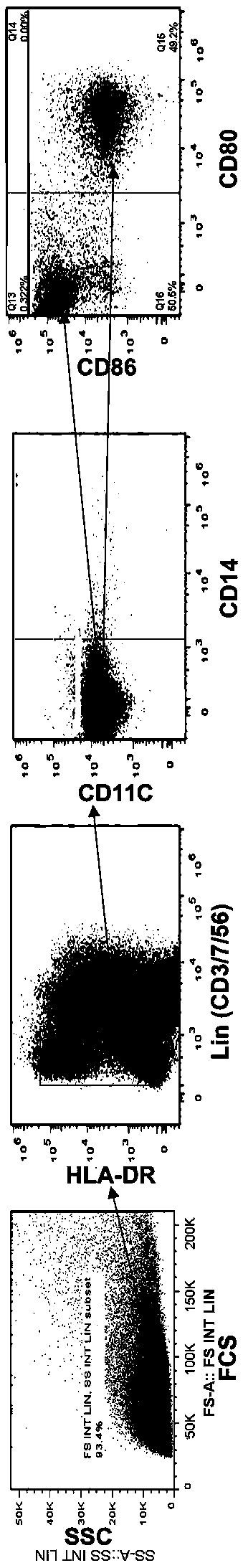
[0032] Example 1: Obtaining antigen-loaded monocyte-derived dendritic cells
[0033] Collect 10ml of peripheral blood from patients with chronic hepatitis B, separate peripheral blood mononuclear cells (PBMC) according to conventional methods, resuspend in 4ml of AIM-V culture medium, and then place them at 37°C, 50% (volume concentration) CO 2 Incubate for 2 hours in an incubator.
[0034] After 2 hours, take it out, shake it gently, and remove the suspended non-adherent cells. Add 4ml of AIM-V medium to the adherent cells, which contains IL-4 (20ng / ml) and GM-CSF (100ng / ml ).
[0035] After 3 days, the adherent cells were supplemented with 2ml of AIM-V culture medium, which contained IL-4 (20ng / ml) and GM-CSF (100ng / ml), and was still placed at 37°C and 50% (volume concentration) CO 2 cultured in an incubator.
[0036] Day 6: Adherent cells: Add 10 μg of hepatitis B vaccine stock solution (Shenzhen Kangtai Company), culture for 24 hours, and obtain functional DC loaded wi...
Embodiment 2
[0038] Example 2: T cell expansion
[0039] The suspended cells (i.e. T cells) obtained in Example 1 were transferred to the AIM-VT75 culture flask containing 4ml, and after the AIM-V medium was washed 3 times (washing liquid volume 2ml / time), 1ug / ml OKT3, 1ug / ml OKT3, 1ug / ml anti-CD28, placed at 37°C, 50% (volume concentration) CO 2 cultured in an incubator.
[0040]Day 2: T cell culture medium was supplemented with 5-10ml of AIM-V culture medium, which contained IL-2 (300IU / ml) and 1.5ml of 20% (mass volume concentration) albumin.
[0041] Day 4: Supplement 5-10ml of AIM‐V culture medium, which contains IL‐2 (300IU / ml) and 1.5ml of 20% (mass volume concentration) albumin )CO 2 Cultured in an incubator until day 8 to obtain proliferating T cells.
Embodiment 3
[0042] Example 3: HBV-specific T cells
[0043] Day 8: The "antigen-loaded functional DC" obtained in Example 1 and the proliferating T cells obtained in Example 2 were mixed and co-cultured.
[0044] Day 10: Supplement 5-10ml of AIM‐V culture medium, which contains IL2 (300IU / ml) and 1.5ml of 20% albumin.
[0045] Day 12: Supplement 5-10ml of AIM‐V culture medium, which contains IL2 (300IU / ml) and 1.5ml of 20% albumin.
[0046] Day 14: Obtain HBV-specific T cells.
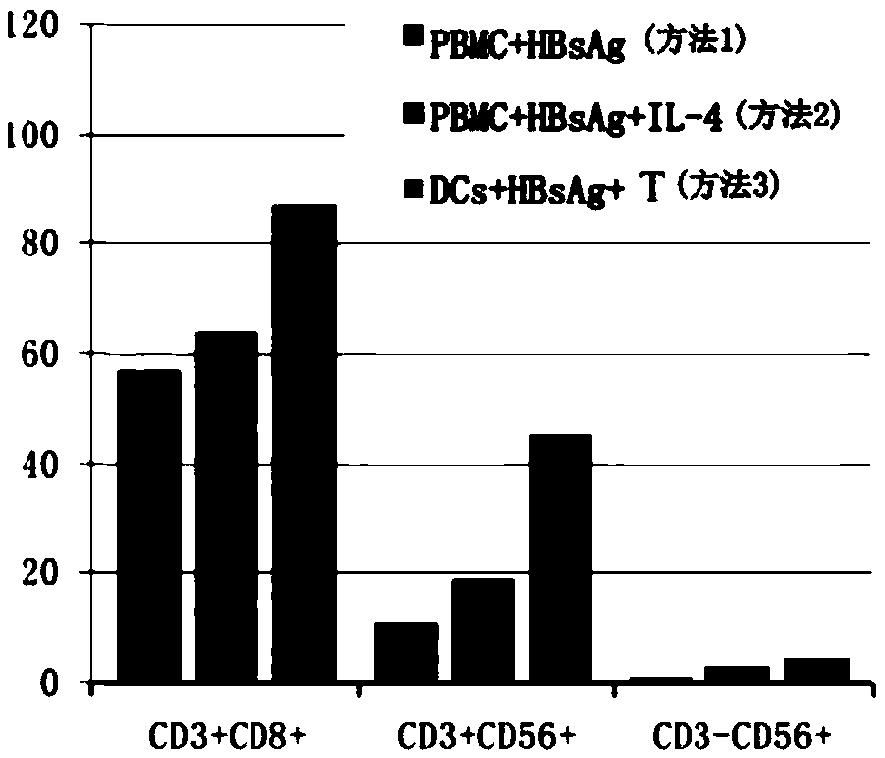
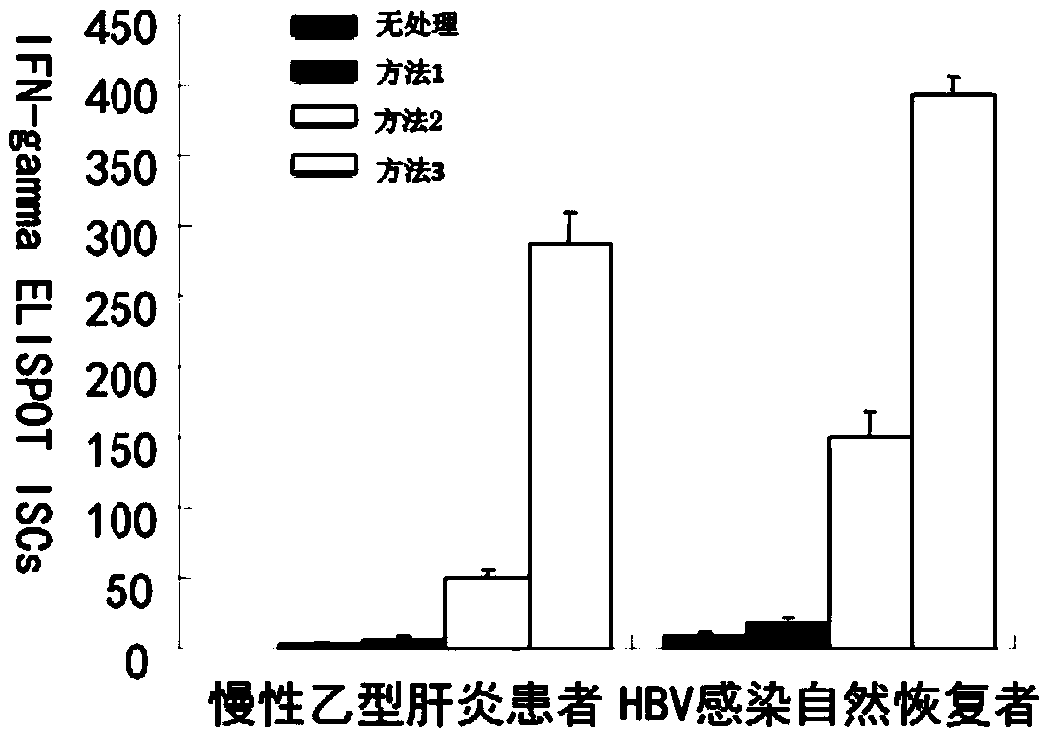
[0047] figure 2 : HBV-sensitized DC co-cultured with T cells can produce more CD8+ cytotoxic T cells.
[0048] Such as figure 2 As shown, the following methods were used to induce PBMCs from chronic HBV-infected patients and healthy people with hepatitis B protective antibodies: method 1: adding hepatitis B vaccine directly to PBMC; method 2: adding hepatitis B vaccine and interleukin 4 to PBMC; method 3 : Co-culture of HBV-sensitized DC and T cells. The results showed that methods 2 and 3 produced higher ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com